The Simplex Process
A Robust Creative Problem-Solving Process
Work through the cycle.
© iStockphoto/centyr
When you're solving business problems, it's all-too-easy easy to skip over important steps in the problem-solving process, meaning that you can miss good solutions, or, worse still, fail to identify the problem correctly in the first place.
One way to prevent this happening is by using the Simplex Process. This powerful step-by-step tool helps you identify and solve problems creatively and effectively. It guides you through each stage of the problem-solving process, from finding the problem to implementing a solution. This helps you ensure that your solutions are creative, robust and well considered.
In this article, we'll look at each step of the Simplex Process. We'll also review some of the tools and resources that will help at each stage.
About the Tool
The Simplex Process was created by Min Basadur, and was popularized in his book, "The Power of Innovation."
It is suitable for problems and projects of any scale. It uses the eight stages shown in Figure 1, below:
Rather than seeing problem-solving as a single straight-line process, Simplex is represented as a continuous cycle.
This means that problem-solving should not stop once a solution has been implemented. Rather, completion and implementation of one cycle of improvement should lead straight into the next.
We'll now look at each step in more detail.
1. Problem Finding
Often, finding the right problem to solve is the most difficult part of the creative process.
So, the first step in using Simplex is to start doing this. When problems exist, you have opportunities for change and improvement. This makes problem finding a valuable skill!
Problems may be obvious. If they're not, they can often be identified using trigger questions like the ones below:
•What would our customers want us to improve? What are they complaining about?
•What could they be doing better if we could help them?
•Who else could we help by using our core competences?
•What small problems do we have which could grow into bigger ones? And where could failures arise in our business process?
•What slows our work or makes it more difficult? What do we often fail to achieve? Where do we have bottlenecks?
•How can we improve quality?
•What are our competitors doing that we could do?
•What is frustrating and irritating to our team?
These questions deal with problems that exist now. It's also useful to try to look into the future. Think about how you expect markets and customers to change over the next few years; the problems you may experience as your organization expands; and social, political and legal changes that may affect it. (Tools such as PEST Analysis will help you to do this.)
It's also worth exploring possible problems from the perspective of the different "actors" in the situation – this is where techniques such as CATWOE can be useful.
At this stage you may not have enough information to define your problem precisely. Don't worry about this until you reach step 3!
2. Fact-Finding
The next stage is to research the problem as fully as possible. This is where you:
•Understand fully how different people perceive the situation.
•Analyze data to see if the problem really exists.
•Explore the best ideas that your competitors have had.
•Understand customers' needs in more detail.
•Know what has already been tried.
•Understand fully any processes, components, services, or technologies that you may want to use.
•Ensure that the benefits of solving the problem will be worth the effort that you'll put into solving it.
With effective fact-finding, you can confirm your view of the situation, and ensure that all future problem-solving is based on an accurate view of reality.
3. Problem Definition
By the time you reach this stage, you should know roughly what the problem is, and you should have a good understanding of the facts relating to it.
From here you need to identify the exact problem or problems that you want to solve.
It's important to solve a problem at the right level. If you ask questions that are too broad, then you'll never have enough resources to answer them effectively. If you ask questions that are too narrow, you may end up fixing the symptoms of a problem, rather than the problem itself.
Min Basadur, who created the Simplex process, suggests saying "Why?" to broaden a question, and "What's stopping you?" to narrow a question.
For example, if your problem is one of trees dying, ask "Why do I want to keep trees healthy?" This might broaden the question to "How can I maintain the quality of our environment?"
A "What's stopping you?" question here could give the answer "I don't know how to control the disease that is killing the tree."
Big problems are normally made up of many smaller ones. This is the stage at which you can use a technique like Drill Down to break the problem down to its component parts. You can also use the 5 Whys Technique, Cause and Effect Analysis and Root Cause Analysis to help get to the root of a problem.
Tip:
A common difficulty during this stage is negative thinking – you or your team might start using phrases such as "We can't..." or "We don't," or "This costs too much." To overcome this, address objections with the phrase "How might we...?" This shifts the focus to creating a solution.
4. Idea Finding
The next stage is to generate as many problem-solving ideas as possible.
Ways of doing this range from asking other people for their opinions, through programmed creativity tools and lateral thinking techniques, to Brainstorming. You should also try to look at the problem from other perspectives. A technique like The Reframing Matrix can help with this.
Don't evaluate or criticize ideas during this stage. Instead, just concentrate on generating ideas. Remember, impractical ideas can often trigger good ones! You can also use the Random Input technique to help you think of some new ideas.
5. Selection and Evaluation
Once you have a number of possible solutions to your problem, it's time to select the best one.
The best solution may be obvious. If it's not, then it's important to think through the criteria that you'll use to select the best idea. Our Decision Making Techniques section lays out a number of good methods for this. Particularly useful techniques include Decision Tree Analysis, Paired Comparison Analysis, and Grid Analysis.
Once you've selected an idea, develop it as far as possible. It's then essential to evaluate it to see if it's good enough to be considered worth using. Here, it's important not to let your ego get in the way of your common sense.
If your idea doesn't offer a big enough benefit, then either see if you can generate more ideas, or restart the whole process. (You can waste years of your life developing creative ideas that no-one wants!)
Techniques to help you to do this include:
•Risk Analysis, which helps you explore where things could go wrong.
•Impact Analysis, which gives you a framework for exploring the full consequences of your decision.
•Force Field Analysis, which helps you explore the pressures for and against change.
•Six Thinking Hats, which helps you explore your decision using a range of valid decision-making styles.
•Use of NPVs and IRRs, which help you ensure that your project is worth running from a financial perspective.
6. Planning
Once you've selected an idea, and are confident that your idea is worthwhile, then it's time to plan its implementation.
Action Plans help you manage simple projects – these lay out the who, what, when, where, why and how of delivering the work.
For larger projects, it's worth using formal project management techniques. By using these, you'll be able to deliver your implementation project efficiently, successfully, and within a sensible time frame.
Where your implementation has an impact on several people or groups of people, it's also worth thinking about change management. Having an appreciation of this will help you assure that people support your project, rather than opposing it or cancelling it.
7. Sell Idea
Up to this stage you may have done all this work on your own or with a small team. Now you'll have to sell the idea to the people who must support it. These may include your boss, investors, or other stakeholders involved with the project.
In selling the project you'll have to address not only its practicalities, but also things such internal politics, hidden fear of change, and so on.
Tip:
You can learn more about how to get support for your ideas with our Bite-Sized Training Session, Sell Your Idea.
8. Action
Finally, after all the creativity and preparation comes action!
This is where all the careful work and planning pays off. Again, if you're implementing a large-scale change or project, you might want to brush up on your change management skills to help ensure that the process is implemented smoothly.
Once the action is firmly under way, return to stage 1, Problem Finding, to continue improving your idea. You can also use the principles of Kaizen to work on continuous improvement.
Key Points:
Simplex is a powerful approach to creative problem-solving. It is suitable for projects and organizations of almost any scale.
The process follows an eight-stage cycle. Upon completion of the eight stages you start it again to find and solve another problem. This helps to ensure continuous improvement.
Stages in the process are:
•Problem finding.
•Fact finding.
•Problem Definition.
•Idea Finding.
•Selection and Evaluation.
•Planning.
•Selling of the Idea.
•Action.
By moving through these stages you ensure that you solve the most significant problems with the best solutions available to you. As such, this process can help you to be intensely creative
Pesquisar neste blogue
sexta-feira, 28 de janeiro de 2011
terça-feira, 25 de janeiro de 2011
Exercícios de memória em idosos
Exercícios mentais para idosos melhoram memória, velocidade de processar informações e de raciocinar em atividades do dia-a-dia
De acordo com estudo, assim como a atividade física traz benefícios para o corpo, exercícios mentais podem ajudar a manter a mente de idosos funcionando melhor, com resultados duradouros.
Idosos que receberam apenas 10 sessões de treinamento mental apresentaram melhoras na memória, no raciocínio e na velocidade de processar informações cinco anos após as sessões, dizem os pesquisadores que conduziram o Advanced Cognitive Training for Independent and Vital Elderly study, ou ACTIVE. Os resultados foram publicados na edição de 20 de dezembro do Journal of the American Medical Association.
Os exercícios mentais foram projetados para melhorar as habilidades de raciocínio de idosos e determinar se estas melhorias poderiam também afetar sua capacidade de seguir corretamente instruções de utilização de medicamentos ou reagir rapidamente aos sinais de tráfego.
"Nossos achados sugerem claramente que pessoas que adotam um programa ativo de treinamento mental na terceira idade podem obter benefícios duradouros desse treinamento," disse o pesquisador Michael Marsiske, professor da University of Florida College of Public Health and Health Professions. "Os resultados positivos do ACTIVE sugerem fortemente que muitos adultos possam aprender e se desenvolver mesmo em idade mais avançada."
Os investigadores descobriram também evidências de "transferência" do treinamento às funções diárias. Comparados àqueles que não receberam treinamento mental, participantes de três grupos de treinamento - memória, velocidade de processar e de raciocinar - relataram menos dificuldade em executar tarefas como cozinhar, usar a medicação e controlar finanças.
"Nós possuíamos aproximadamente 25 anos de conhecimento, antes deste estudo, que sugeria que as habilidades de raciocínio de idosos poderiam ser treinadas, mas nós não sabíamos se estes ganhos mentais afetariam habilidades na prática diária" disse Marsiske. "Neste estudo nós obtivemos evidências de que treinar as funções mentais básicas pode também melhorar a habilidade dos idosos em executar tarefas de sua rotina."
Realizado entre 1998 e 2004, o estudo ACTIVE é o primeiro realizado em grande escala alternando treinamento cognitivo em idosos saudáveis. Financiado pelo National Institute on Aging e pelo National Institute of Nursing Research, o estudo envolveu 2.802 idosos de 65 a 96 anos, os quais foram divididos em grupos para receber o treinamento em memória, raciocínio ou velocidade de processar, em 10 sessões de 90 minutos cada, durante seis semanas. O grupo de controle não recebeu treinamento.
Os idosos do grupo de treinamento da memória aprenderam estratégias para recordar listas de palavra e seqüências de itens, material textual e idéias principais e detalhes de histórias. Os participantes do grupo de raciocínio receberam instrução em como resolver problemas que seguem padrões, uma habilidade útil em tarefas como ler uma programação de horário de ônibus ou preencher um formulário. O treinamento de velocidade de processamento foi feito com programas para computadores que focalizaram a habilidade para identificar rapidamente e encontrar informações visuais, habilidades usadas ao procurar números na lista de telefone ou ao reagir aos sinais de tráfego.
Quando imediatamente testados depois do período de treinamento, 87% dos participantes do treinamento de velocidade, 74% do de raciocínio e 26% do de memória mostraram melhoria na confiança em suas respectivas habilidades mentais.
Em relatórios posteriores, os investigadores encontraram que as melhorias persistiram por dois anos após o treinamento, particularmente para os idosos que foram sorteados para receber treinamento de reforço de um a três anos após o treinamento original.
Fonte: http://www.news.med.br/p/exercicios+mentais+para+idosos+melh-10444.html
De acordo com estudo, assim como a atividade física traz benefícios para o corpo, exercícios mentais podem ajudar a manter a mente de idosos funcionando melhor, com resultados duradouros.
Idosos que receberam apenas 10 sessões de treinamento mental apresentaram melhoras na memória, no raciocínio e na velocidade de processar informações cinco anos após as sessões, dizem os pesquisadores que conduziram o Advanced Cognitive Training for Independent and Vital Elderly study, ou ACTIVE. Os resultados foram publicados na edição de 20 de dezembro do Journal of the American Medical Association.
Os exercícios mentais foram projetados para melhorar as habilidades de raciocínio de idosos e determinar se estas melhorias poderiam também afetar sua capacidade de seguir corretamente instruções de utilização de medicamentos ou reagir rapidamente aos sinais de tráfego.
"Nossos achados sugerem claramente que pessoas que adotam um programa ativo de treinamento mental na terceira idade podem obter benefícios duradouros desse treinamento," disse o pesquisador Michael Marsiske, professor da University of Florida College of Public Health and Health Professions. "Os resultados positivos do ACTIVE sugerem fortemente que muitos adultos possam aprender e se desenvolver mesmo em idade mais avançada."
Os investigadores descobriram também evidências de "transferência" do treinamento às funções diárias. Comparados àqueles que não receberam treinamento mental, participantes de três grupos de treinamento - memória, velocidade de processar e de raciocinar - relataram menos dificuldade em executar tarefas como cozinhar, usar a medicação e controlar finanças.
"Nós possuíamos aproximadamente 25 anos de conhecimento, antes deste estudo, que sugeria que as habilidades de raciocínio de idosos poderiam ser treinadas, mas nós não sabíamos se estes ganhos mentais afetariam habilidades na prática diária" disse Marsiske. "Neste estudo nós obtivemos evidências de que treinar as funções mentais básicas pode também melhorar a habilidade dos idosos em executar tarefas de sua rotina."
Realizado entre 1998 e 2004, o estudo ACTIVE é o primeiro realizado em grande escala alternando treinamento cognitivo em idosos saudáveis. Financiado pelo National Institute on Aging e pelo National Institute of Nursing Research, o estudo envolveu 2.802 idosos de 65 a 96 anos, os quais foram divididos em grupos para receber o treinamento em memória, raciocínio ou velocidade de processar, em 10 sessões de 90 minutos cada, durante seis semanas. O grupo de controle não recebeu treinamento.
Os idosos do grupo de treinamento da memória aprenderam estratégias para recordar listas de palavra e seqüências de itens, material textual e idéias principais e detalhes de histórias. Os participantes do grupo de raciocínio receberam instrução em como resolver problemas que seguem padrões, uma habilidade útil em tarefas como ler uma programação de horário de ônibus ou preencher um formulário. O treinamento de velocidade de processamento foi feito com programas para computadores que focalizaram a habilidade para identificar rapidamente e encontrar informações visuais, habilidades usadas ao procurar números na lista de telefone ou ao reagir aos sinais de tráfego.
Quando imediatamente testados depois do período de treinamento, 87% dos participantes do treinamento de velocidade, 74% do de raciocínio e 26% do de memória mostraram melhoria na confiança em suas respectivas habilidades mentais.
Em relatórios posteriores, os investigadores encontraram que as melhorias persistiram por dois anos após o treinamento, particularmente para os idosos que foram sorteados para receber treinamento de reforço de um a três anos após o treinamento original.
Fonte: http://www.news.med.br/p/exercicios+mentais+para+idosos+melh-10444.html
quinta-feira, 20 de janeiro de 2011
Doença de Alzheimer e Fisioterapia
A doença de Alzheimer é uma doença neuropsiquiátrica progressiva do envelhecimento encontradas em adultos de meia-idade e, particularmente, em mais velhos, que afeta a substância cerebral e é caracterizada pela perda inexorável da função cognitiva, bem como distúrbios afetivos e comportamentais. A principal causa de demência em adultos com mais de 60 anos, o mal de Alzheimer, é responsável por alterações de comportamento, de memória e de pensamento (1).
A doença se caracteriza pela morte gradual de neurônios, as células nervosas do cérebro. As causas desse desastre são pouco conhecidas. Sabe-se que ele está relacionada a um acúmulo de duas proteínas, a beta-amilóide (2).
Doença de Alzheimer
A doença de Alzheimer caracteriza-se pela atrofia do córtex cerebral. O processo geralmente é difuso, mas pode ser mais grave nos lobos frontal, parietal e temporal. O grau de atrofia varia(3).
O envelhecimento normal do cérebro também se acompanha de atrofia, há uma superposição no grau de atrofia do cérebro de pacientes idosos com Alzheimer e pessoas da mesma idade afetadas pela doença. Ao exame microscópio, há perda tanto de neurônio como de neurópilo no córtex e, ocasionalmente, se observa uma desmielinização secundária na substância branca subcortical. Com o uso da morfometria quantitativa, a maior perda é a de grandes neurônios corticais (4).
Os achados mais característicos são placas senis e emaranhadas neurofibrilares argentofílicos. A placa senil é encontrada em todo o córtex e hipocampo e o número de placas por campo microscópio correlaciona-se com o grau de perda intelectual. Emaranhados neurofibrilares são estruturas fibrilares intracitoplasmáticas neuronais (5).
A degeneração e a perda neuronal são gerais, embora especialmente acentuadas nas células piramidais do hipocampo e células piramidais grandes no córtex associativo. A degeneração aparece cedo no núcleo basal de Meynert e mais tarde nos lócus coeruleos. A patologia inclui a presença de desarranjos neurofibrilares, placas neuríticas e degeneração granulovacuolar. Os desarranjos são massas intraneurofibrilares de filamentos citoplásticos (4).
Epidemiologia
Nos EUA, a prevalência da doença de Alzheimer em pessoas de 65 anos de idade ou mais é estimada como sendo de 10,3 %, elevando-se para 47 % naquelas acima de 80 anos. Até 2,6 % das pessoas acima dos 65 anos vem apresentar a Doença de Alzheimer anualmente. A freqüência varia pouco por sexo ou grupo étnico(1).
Etiologia
Embora a causa da Doença de Alzheimer não tenha sido estabelecida, há fortes suspeitas de uma base genética. A concordância para Doença de Alzheimer em gêmeos homozigóticos é maior que em gêmeos dizigóticos (3).
Agentes infecciosos e contaminantes ambientais, incluindo vírus lentos e metais (por exemplo o alumínio) são fatores etiológicos suspeitos. Os papéis destes fatores ainda não foram comprovados; no entanto, estes fatores ambientais estão sendo submetidos à investigação ativa. O envelhecimento normal está associado com decréscimo de alguns neurotransmissores, bem como com alguns dos achados neuropatológicos da doença. Portanto, surgiu a questão sobre se a doença seria devido a uma aceleração das alterações normais do envelhecimento (3).
As lesões crânio encefálicas, baixos níveis de instrução e síndrome de Down em um parente em primeiro grau também se associa a maior risco de doença de Alzheimer (3).
O quadro clínico da doença de Alzheimer caracteriza-se por um declínio insidioso, progressivo da memória e de outras funções corticais como linguagem, conceito, julgamento, habilidades visuo-espaciais. Progressivamente instalam-se alterações intelectuais e de esfera afetiva, mas sobressaem os distúrbios de funções simbólicas: apraxias, agnosias (perda da capacidade de interpretar o que vê, ouve ou sente) e afasias. Pode ocorrer manifestação psicótica, como delírios e crises convulsivas (6). Apresentam geralmente delírios de infidelidade conjugal e roubos. Esses pacientes podem vagar, marchar, abrir e fechar gavetas rapidamente, bem como fazer as mesmas perguntas várias vezes. Anormalidades do ciclo sono-vigília podem tornar-se evidentes, como ficar acordado durante a noite, por pensar que ainda é dia claro (3). Alguns pacientes desenvolvem uma marcha arrastada, com rigidez muscular generalizada associada à lentidão e inadequação do movimento. Possuem com frequência um aspecto parkinsoniano, mas raramente tem tremor em repouso rápido e rítmico. Podem evidenciar reflexos tendíneos hiperativos e reflexos de sucção e muxoxo. Abalos mioclônicos (contrações bruscas breves de vários músculos ou de todo o corpo) podem ocorrer espontaneamente ou em respostas a estímulos físicos ou auditivos (2).
As perdas cognitivas aumentam e o indivíduo evolui até ficar totalmente dependente de outros para a execução de suas atividades mais básicas. Em estágios mais avançados passa a não se alimentar, engasgar-se com a comida e saliva, o vocabulário fica restrito a poucas palavras, perde a capacidade de sorrir, sustentar a cabeça, fica acamado e a morte sobrevém em conseqüência de complicações como pneumonia, desidratação ou sepsis (6).
A demência senil do tipo Alzheimer pode ainda ser subdividida de acordo com o estágio clínico, mas existe grande variabilidade e a evolução dos estágios freqüentemente não é tão ordenada como se poderia deduzir da descrição que vem
Estágio Inicial: perda da memória recente, incapacidade de aprender e reter informações novas, problemas de linguagem, labilidade de humor e, possivelmente, alterações de personalidade. Os pacientes podem apresentar dificuldade progressiva para desempenhar as atividades de vida diária. Irritabilidade, hostilidade e agitação podem ocorrer como resposta à perda de controle e de memória. O estágio inicial, no entanto, pode não comprometer a sociabilidade (7).
Estágio Intermediário: completamente incapaz de aprender e lembrar de informações novas. Os pacientes se perdem constantemente, frequentemente a ponto de conseguirem encontrar o seu próprio quarto ou banheiro. Embora continuem a deambular, estão em risco significativo de quedas ou acidentes secundários à confusão. O paciente pode precisar de assistência nas AVDs. A desorganização comportamental ocorre na forma de perambulação, agitação, hostilidade, falta de cooperação ou agressividade física. Neste estágio, o paciente já perdeu todo o senso de tempo e lugar (7).
Estágio grave ou terminal: incapaz de andar, totalmente incontinente e incapaz de desempenhar qualquer AVD. Podem ser incapazes de deglutir e podem necessitar de alimentação por sonda nasogastrica. Estão em risco de pneumonia, desnutrição e necrose da pele por pressão (7).
A evolução da doença é gradual, e não rápida ou fulminante; existe um declínio constante, embora os sintomas em alguns pacientes pareçam se estabilizar durante algum tempo. Não ocorrem sinais motores ou outros sinais neurológicos focais ate tardiamente na doença (1). A duração típica da doença é de 8 a 10 anos, mas a evolução varia de 1 a 25 anos. Por motivos desconhecidos, alguns pacientes com Alzheimer evidenciam um declínio gradual e lento da função, enquanto outros têm platôs prolongados sem deterioração importante (2). O estágio final da doença de Alzheimer é coma e morte. (1)
Dentre as principais características estão (7):
Perda de memória: pode ter conseqüência na vida diária, de muitas maneiras, conduzindo a problemas de comunicação, riscos de segurança e problemas de comportamento.
Memória episódica: é a memória que as pessoas têm de episódios da sua vida, passando do mais mundano ao mais pessoalmente significativo.
Memória semântica: esta categoria abrange a memória do significado das palavras, como por exemplo, uma flor ou um cão.
Memória de procedimento: esta é a memória de como conduzir os nossos atos quer física como mentalmente, por exemplo, como usar uma faca e um garfo, ou jogar xadrez.
Apraxia: é o termo usado para descreve a incapacidade para efetuar movimentos voluntários e propositados, apesar do fato da força muscular, da sensibilidade e da coordenação estarem intactas.
Afasia: é o termo utilizado para descrever a dificuldade ou perda de capacidade para falar, ou compreender a linguagem falada, escrita ou gestual, em resultado de uma lesão do respectivo centro nervoso.
Agnosia: é o termo utilizado para descrever a perda de capacidade para reconhecer o que são os objetos, e para que servem.
Comunicação: As pessoas com doença de Alzheimer tem dificuldades na emissão e na compreensão da linguagem, o que, por sua vez, leva a outros problemas.
Mudança de personalidade: uma pessoa que tenha sido sempre calma, educada e afável pode comportar-se de uma forma agressiva e doentia. São comuns as mudanças bruscas e freqüentes de humor.
Mudanças físicas: a perda de peso pode ocorrer, redução de massa muscular, escaras de decúbito, infecções, pneumonia.
A evolução é progressiva, inevitavelmente em incapacidade completa e morte. Ás vezes há estabilizações, durante as quais o distúrbio cognitivo permanece inalterado por um ou dois anos, mas depois a progressão é retomada. A duração é geralmente entre 4 a 10 anos, com extremos de menos de 1 ano e a mais de 20 anos (8).
Tratamento
Por enquanto, não existe tratamento preventivo ou curativo para a doença de Alzheimer. Existe uma série de medicamentos que ajudam a aliviar alguns sintomas, tais como agitação, ansiedade, depressão, confusão e insônia. Infelizmente, estes medicamentos são apenas eficazes para um número limitado de doentes, apenas por um breve período de tempo e podem causar efeitos secundários indesejados. Por isso, aconselha-se geralmente a evitar a medicação, a menos que seja realmente necessária (7).
Descobriu-se que os doentes que têm a doença de Alzheimer têm níveis reduzidos de acetilcolina, um neurotransmissor (substância química responsável pela transmissão de mensagens de uma célula para outra) que intervém nos processos da memória (9).
Foram introduzidos, em alguns países, determinados medicamentos que inibem a enzima responsável pela destruição da acetilcolina. (4).
Os resultados no tratamento da doença são difíceis e frustrantes, pois não há medidas específicas e a ênfase primária é o alívio em longo prazo dos problemas comportamentais e neurológicos associados (2).
O tratamento é multimodal, desenvolvido e modificado com a progressão da doença, podendo ser obtido através da intervenção farmacológica (visando à fisiopatologia e sintomas da doença como ilusões e distúrbios do sono) e da intervenção comportamentais (melhora sintomas específicos bem como as AVDs). Atualmente os agentes farmacológicos empregados no tratamento visam inibir a acetilcolinesterase para aumentar os níveis de acetilcolina diminuídos no cérebro dos pacientes com Alzheimer. Os inibidores utilizados atualmente (utilizados apenas em doenças com déficit colinérgico como Alzheimer) são: Tacrina (melhora apatia e ansiedade), Donepezil (1).
Fisioterapia
Designa a habilitar o indivíduo comprometido funcionalmente, a novamente desempenhar suas AVDs, da melhor maneira e pelo menor tempo possível, com mais autonomia. Os principais aspectos da assistência são preventivos, elaborados para manter o indivíduo mais ativo e independente possível. No maior grau que for possível, a atividade deve ser encorajada para manter a força, ADM e estado de alerta (9).
Promover somente a ajuda que é absolutamente necessária, com os pacientes continuando a realizar sozinhos máximo de AVDs possível; o paciente precisa ser capaz de lembrar o comando, identificar o objeto (exemplo, escova de dente), e realizar a ação motora (8).
O processo de reabilitação também inclui a realização de modificações ambientais necessárias para segurança do paciente e de modo que esse possa viver em um ambiente o mais aberto possível (8). Quedas podem ser devido a riscos ambientais o mais (iluminação inadequada, pisos escorregadios, tapetes soltos, etc). Tais riscos devem ser evitados. Os ambientes devem manter um apresentação familiar (não mudar a disposição da mobília desnecessariamente) e barras presas na parede e corrimãos devem ser instalados nos locais necessários (10).
As técnicas de fisioterapia serão as mesmas que usamos nas pessoas de terceira idade que não apresentam demência, mas a maneira de aborda-las exige habilidade especial. O paciente pode não aparecer interessado em saber como a falta de flexibilidade articular, a fraqueza muscular ou o edema poderão afeta-lo; não tem condições de entender a relação que existe. O preparo antecipado dos planos de terapêutica impede que o fisioterapeuta pareça indeciso. O preparo de explicações claras e simples darão melhores resultados. A instrução deverá ser repetida da mesma forma; o emprego das palavras diferentes poderá confundir o paciente. Não é aconselhável entrar em conversas desnecessárias, pois podem desnorteá-lo mais ainda.Lançar mãos de gestos e sinais físicos para esclarecer e reforçar as instruções exigentes (9).
São comuns as alterações de sensibilidade, por isso evitar atração, a eletroterapia e os equipamentos que exigem colaboração de participação subjetiva. O calor somente deve ser usado naqueles capazes de reconhecer e dizer quanto é demasiado quente (9).
O estabelecimento de metas realizáveis no tocante à mobilidade é muito útil. Mesmo as realizações mais modestas merecem ser recompensadas por parabéns e pelo interesse que o fisioterapeuta demonstra pela pessoa (9).
A meta de reabilitação precisa ser redefinida para assegurar que o paciente permaneça seguro, independente, e capaz de realizar atividades da vida diária e atividades instrumentais da vida pelo máximo de tempo que for razoável (8).
O processo de reabilitação pode começar enquanto o trabalho de diagnóstico está ainda sendo feito. Isso pode tornar a forma de treinamento básico em atividades da vida diária. Isso inclui o treinamento de atendentes para a assistência de paciente e a reabilitação de modificações ambientais necessárias para a segurança da pessoa confusa de modo que essa possa viver em um ambiente o mais aberto possível. Uma vez o diagnóstico tenha sido estabelecido, pode ser feito o planejamento cuidadoso para a assistência.
Nos estágios iniciais e médios da doença, a intervenção fisioterapêutica geralmente pode prolongar a habilidade de movimentar-se com facilidade (10).
É importante observar que inicialmente a intervenção fisioterapêutica pode ser potencialmente envolvida com a facilitação do planejamento motor e planejamento para compensação de perdas nas atividades de vida diária. A habilidade para andar é perdida mais tarde, e outros estudos relatam níveis comparáveis de comprometimento (7).
A intervenção fisioterapêutica para assistir o paciente e treinar os atendentes envolve a facilitação dos movimentos e planejamentos motor e o desenvolvimento ou refinamento de pistas ambientais e cognitivas para ajudar a realizar tarefas complexas (10).
O comprometimento cognitivo é o fator limitante. A análise ajuda o atendente a prover somente a ajuda que é absolutamente necessária, com os pacientes continuando a realizar sozinhos o máximo de AVDS possível; por exemplo, para escovar os dentes, o paciente precisa ser capaz de lembrar o comando, identificar a escova de dentes, e realizar a ação motora. O paciente pode somente precisar de ajuda de alguém que coloque a escova em sua mão e a guie lentamente para a boca para que seja capaz de escovar os dentes (7).
A primeira meta da reabilitação é criar um ambiente (emocional e físico) que dê suporte (trabalhar ativamente para compensar as perdas cognitivas específicas dos pacientes na medida em que forem ocorrendo gradualmente). A meta final é ajudar os pacientes a sentirem que eles são capazes, de modo que continuem a tentar fazer por si próprios as coisas que possam fazer com segurança, independentemente de permanecerem em sua casa ou estarem vivendo em uma instituição (9).
Em cada sessão os movimentos precisam ser equilibrados com períodos adequados de repouso para assegurar que o paciente não atingirá o ponto de fadiga e exaustão. São preferíveis freqüentes e breves períodos de atividade física (10).
O fisioterapeuta pode contribuir muito com a melhora da qualidade de vida do paciente e da família (7).
Objetivos da reabilitação fisioterapêutica (7,8,9):
- Diminuir a progressão e efeitos dos sintomas da doença,
- Evitar ou diminuir complicações e deformidades,
- Manter as capacidades funcionais do paciente (sistema cardiorrespiratório),
- Manter ou devolver a ADM funcional das articulações,
- Evitar contraturas e encurtamento musculares (sequelas da imobilização no leito),
- Evitar a atrofia por desuso e fraqueza muscular,
- Incentivar e promover o funcionamento motor e mobilidade,
- Orientação sobre as posturas corretas,
- Treino do padrão da marcha,
- Trabalhar os padrões do funcionamento sistema respiratório (fala, respiração, expansão e mobilidade torácica),
- Manter ou recuperar a independência funcional nas atividades de vida diária.
Consiste basicamente na fisioterapia motora, que engloba desde exercícios ativos, passivos, auto-assistidos, contra-resistência, isométricos, metabólicos, isotônicos, ou seja, qualquer tipo de movimento é bem vindo no tratamento. Quando chega a uma segunda fase esta patologia, deve-se estimular atividades para que o paciente não fique o tempo inteiro na cama, evitando assim escaras de decúbito, comumente relatadas. Alongamentos e atividades de relaxamento podem ser realizados (10).
Outras modalidades de tratamento são eficazes, como a parte de exercícios para o condicionamento aeróbico, como estimular o paciente a realizar caminhadas, tanto por percursos diferentes, ou dependendo pelo mesmo percurso, estimulando assim concomitantemente a memória do paciente. Bicicletas estacionárias também são de grande valia, assim como se possíveis trabalhos de hidroterapia (9).
Exercícios para propriocepção e equilíbrio são fundamentais para a desenvoltura do paciente, como exercícios com bastões, bola, descarga de peso gradual, andadores, percursos (10).
Pode realizar atividades em que se estimule o raciocínio do paciente, como atividades de escrever, decorar palavras, nomear objetos, que levam a um estímulo da memória (7).
Porém a principal atitude a ser tomada é a companhia que este paciente precisa, nunca deixa-lo no abandono, por isso a conversa com familiares sobre o assunto é importante, para que assim este paciente possa conviver normalmente num meio familiar, eliminando o risco de passar o resto de sua vida num asilo (6).
Conclusão
Existe uma busca de uma melhor qualidade de vida para os pacientes e dos vários tipos de tratamento para a doença de Alzheimer, com o objetivo de amenizar os sintomas. Porém enquanto não for descoberta a etiologia dessa patologia, torna-se difícil chegar á cura dessa doença.
A fisioterapia busca melhoria na qualidade de vida do paciente, sendo que o paciente com Alzheimer necessita de uma reabilitação global, envolvendo uma equipe multidisciplinar, e que a fisioterapia tem um papel fundamental tanto na reabilitação motora quanto no retorno as relações interpessoais e na obtenção de independência por parte do paciente.
Referência
1. Goldman, L.; Bennett, J.: et al. Cecil: Tratado de Medicina Interna. 2 Ed. Rio Janeiro: Guanabarra Koogan 2001.v2.
2. Harrison, T. R. Medicina Interna. 5 Ed. Rio de Janeiro: Mc Graw Hill, 2002 v2. 3. Rowland, Lewis P. et al. Merrit: Tratado de Neurologia. 9 Ed. Rio de Janeiro: Guanabara Koogan, 1997.
4. Abrams, William B.; Berkow, Robert. Manual Merck de Geriatria. 1 Ed. São Paulo: Roca, 1994.
5. Papaléo Netto, Matheus. Gerontologia: A velhice e o envelhecimento em visão globalizada. São Paulo: Atheneu, 1996.
6. Serro Azul, Luis G. Carvalho Filho, Eurico T.; Décourt, Luiz. Clínica do Indivíduo Idoso. Rio de Janeiro: Guanabara, 1981.
7. O´sullivan S.B., Schimitz T.J., Fisioterapia: Avaliação e Tratamentos. 3 Ed. São Paulo. Manole, 2003.
8. Umphred, Darcy Ann. Fisioterapia Neurológica. 2.ed. São Paulo: Manole, 1994.
9. Compton, Ann et al., Fisioterapia na terceira idade. São Paulo: Santos, 2002.
10. Kottke, Frederic J.; Lehmann, Justus F. Tratado de Medicina Física e Reabilitação de Krusen.. 4 Ed. São Paulo: Manole ,1994.
sexta-feira, 14 de janeiro de 2011
Avanços recentes na Reabilitação de Proteses Totais do Joelho
Following Primary Total Knee Arthroplasty
JUSTINE NAYLOR, ALISON HARMER AND RICHARD WALKER
CASE REPORT
Mrs JM, a 70 year old female, presented pre-operatively with severe tri-compartmental osteoarthritis (OA) of her right knee. On examination, she was obese (Body Mass Index (BMI) 30.8), walked with a varus thrust and a marked limp on the right, and used a walking stick. Her gait, lower limb strength, and range of motion (ROM) profiles were as follows:
Gait speed:
– Timed up-and-go (TUG) – 15 seconds.
– Timed 15-m walk – 21 seconds (0.71 m/s).
– 6-min. Walk Test (6 MWT), 322m, limited by knee pain (right > left).
Isometric strength at 90◦:
– Knee extensors: Right, 106 Newtons; Left, 150 Newtons.
– Knee flexors: Right, 58 Newtons; Left, 100 Newtons.
Knee range of motion (ROM) (passive, supine):
– Right=−10◦ to 100◦; Left=−5 ◦ to 105◦.
Symptomatically, Mrs JM reported high pain (13/20), stiffness (5.8/5), and difficulty (45.5/68) scores on the WOMAC1 subscales, and poor bodily pain (30/100) and physical function (26.6/100) scores on the SF-362 domains.
In terms of Mrs JM’s medical history, she reported bilateral knee OA (right > left) of idiopathic origin of eight year’s duration. She suffered from hypertension (which was controlled), ischaemic heart disease (IHD), and demonstrated poorly controlled type 2 diabetes mellitus (HbA1c (glycosylated haemoglobin) 8.2 %) of seven years’ duration. Consequently, her American Society of Anesthesiologists (ASA) anaesthetic risk score was estimated as II. Consequent to her multiple co-morbidity status, her medication usewas extensive; for her pain management in particular, a poly-pharmacy approach was evident:
1 Western Ontario & MacMaster Universities Osteoarthritis Index (low scores indicating better status).
2 Medical Outcome Study, Short Form-36 Health related quality of life scale (high scores indicating better
Carvedilol, 25 mg daily.
Glyceryl trinitrate, patch 25 mg daily.
Metformin, 1 g bd.
Paracetamol, prn.
Celecoxib, 200 mg daily.
Glucosamine and chondroitin sulphate.
Her haemoglobin concentration (Hb) was noted to be 139 g/l.
As part of routine anaesthetic work-up.
Socially, Mrs JM lived with her spouse in a house with 18 stairs. She had ceased recreational lawn bowls six months prior to her presentation due to pain and giving way in her right leg. She was a pensioner, reporting a low income level throughout her family life, and the highest level of education attainedwas primary (elementary) level.
INTRODUCTION
The benefits of total knee arthroplasty (TKA) for the individual with arthritis are perceived relatively quickly (usually within three to six months) and are generally pluralistic, including improvements in pain, ROM, knee stability, mobility, function, and health-related quality of life (HRQoL) (Aarons et al. 1996 A; Ethgen et al.
2004 A; Fortin et al. 2002 A; March et al. 1999 A; March et al. 2004 A; McAuley et al. 2002 A; Naylor et al. 2006a A; Pierson et al. 2003 A; Salmon et al. 2001 A; Van Essen et al. 1998 A). Consequently, TKA is estimated to be a highly cost-effective treatment option for severe arthritis (Segal et al. 2004 A). Largely ignored in costbenefit calculations, however, are the costs associated with ongoing (post-acute care)
rehabilitation. Such costs can indirectly be appreciated via the findings of March et al. (2004 A), who reported that the average number of out-patient physiotherapy visits by primary TKA patients was 10 in the first post-operative year, exceeding the average number of patient visits to any other health professional. This, of course, was in addition to any acute in-patient rehabilitation provided during the in-patient period
(an average of 12 days) and, for many (33 %), treatment in a rehabilitation facility.
We anticipate that the findings by March et al. are readily generalised as we have observed that referral to ongoing physiotherapy post-TKA is fairly routine in Australia, with out-patient based treatment predominating (Naylor et al. 2006bA). Our findings, obtained through a nationwide survey of TKA rehabilitation providers, echo earlier observations by Lingard et al. (2000A), who reported the frequent utilisation of ongoing physiotherapy post-TKA in the UK, Australia and the US, with the latter tending to rely more on in-patient services. Given that the numbers of TKA procedures have doubled in these same countries over the last decade (Australian Orthopaedic Association National Joint Replacement Registry 2004 A; Dixon et al. 2004 A; Skinner et al. 2003 A), the volumes of patients potentially requiring ongoing rehabilitation to supplement surgery must also have increased. Anecdotally, in Australia at least, there is a perception that the increased surgical throughput has not been accompanied by increases or appropriate increases in the availability of downstream (ward-based and rehabilitative) resources. This must translate at some point into a time-squeeze at the therapist-patient interface and access-block for rehabilitation services. For these reasons, the need to understand the costs and benefits of rehabilitation should be an urgent priority for health systems worldwide. Osteoarthritis (OA), the leading precipitant for TKA, is associated with significant loss of lower limb muscle strength (Fransen et al. 2003 A; Gur et al. 2002 A), walking speed (Gur et al. 2002 A; Lamb & Frost 2003 A), and function (Fransen et al. 2001 A). Exercise programmes involving patients with OA have repeatedly been shown to elicit significant yet small improvements in these parameters within relatively short time frames (for example, at two months) (see reviews by Bischoff & Roos 2003 R; Fransen et al. 2001 R). In contrast, TKA – a procedure typically reserved for recalcitrant arthritis – does not guarantee immediate improvements in these same parameters. Though significant improvements do occur early, several cross-sectional (Berth et al. 2002 A; Mizner et al. 2003 A; Walsh et al. 1998 A) and longitudinal (Benedetti et al. 2003 A; Lamb & Frost 2003 A; Lorentzen et al. 1999 A; Ouellet & Moffet 2002 A; Salmon et al. 2001 A) studies reveal shortfalls in gait, strength, and quality of life, compared to age-matched controls, several months to years after surgery. The argument for ongoing rehabilitation following TKA, therefore, is based on the following related contentions:
That age-predicted norms for muscle function, gait patterns, and physical activity levels are not spontaneously or completely achieved post-surgery, and;
That short-term exposure to prescribed interventions or physical activities will facilitate more complete recovery.
Given that the provision of acute and ongoing physiotherapeutic rehabilitation appears to be standard care across several countries, it is staggering to realise that the evidencebase which underpins rehabilitation in this area is tenuous. While there are considerable bodies of work supporting some, but not all, physiotherapeutic interventions in the acute ward phase, there is comparatively little evidence to support the various modes of ongoing rehabilitation offered either in the community or in rehabilitation wards. The trials that have been conducted (Frost et al. 2002 A; Kramer et al. 2003 A; Moffet et al. 2004; Rajan et al. 2004 A) all compared one mode of ongoing physiotherapy to another and did not include a true non-interventional control. Thus, the contribution of rehabilitation per se to the overall recovery process is uncertain.The lack of definitive evidence is problematic for policy makers worldwide, as health service providers are increasingly required to justify the high costs of health care, while the demand for services (in this case, rehabilitation) is increasing through sheer volume alone. Furthermore, the lack of evidence is problematic at the coalface, given that variation in practice is likely to be (Roos 2003C), and has been observed to be (Naylor et al. 2006b A), the rule, further undermining our capacity to identify best practice.
This chapter addresses questions concerning the efficacy of various acute physiotherapeutic interventions and longer-term rehabilitative strategies Mrs JM may be exposed to through her journey of recovery. Questions concerning the impact of prosthesis type or specific surgical choices on the potential to rehabilitate or the mode of rehabilitation required are also briefly addressed. Mrs JM presents fairly typically for an elderly patient awaiting TKA for severe knee OA (Ackerman et al. 2005 A; Bozic et al. 2005 A; Heck et al. 1998 A; March et al. 2004 A; Mizner et al. 2003 A; Naylor et al. 2006a A; Ouellet & Moffet 2002 A). Notably, the measured variables are frequently utilised and recommended for the evaluation of OA and TKA (Bellamy et al. 1988 A; Ethgen et al. 2004 R; Fransen et al. 2003 A; Kennedy et al. 2005 A; March et al. 1999 A; March et al. 2004 A; Ouellet & Moffet 2002 A; Petterson et al. 2003 A). Compared to norm data or age-matched controls (see Table 1),
Table 1. Normative or age-matched physical and health-related quality of life data
Australian Age-Matched
Norm Data Control Data
Physical Function
SF-36 Physical Function 65.2 1 —
WOMAC Physical Function NA —
Walking Speeds
Timed up-and-go (sec) — 8–11 2,3,4
15-m walk (m/sec) — 1.33–1.84 2,5
6-minute walk (m) — 448 2
Isometric Muscle Strength
Knee Extensors (N) — 225 (sd 49)6
Knee Flexors (N) — 139 (36)6
Health-Related Quality of life
SF-36 General Health 64.1 —
SF-36 Vitality 60 —
SF-36 Mental Health 75.3 —
Knee Range of Motion
Total — 143◦4
Pain Scores
SF-36 Bodily Pain 69 —
WOMAC Pain NA —
Legend: 1National Health Survey SF-36 Population Norms, ABS 1995 (unstandardised mean scores, female); 2Steffen et al. 2002 A; 3Ouellet & Moffet 2002 A; 4Shumway-Cook et al. 2000 A; 5Walsh et al. 1998 A; 6Fransen et al. 2003A; NA=not available at time of publication (Australian data). Normative data from large population sets are provided where available; otherwise, age-matched data, sourced from relevant osteoarthritis or knee replacement trials, are cited.
The reported daily consumption of analgesic and anti-inflammatory medications is consistent with the high pain scores, and the use of a walking aid is somewhat typical for degenerative joint disease. It is important to note that our own experiences indicate the analgesic, anti-inflammatory, and walking aid profiles are not, in isolation, reliableindicators of severity or improvement, as behavioural factors greatly influence their use.
The patient’s co-morbidity profile is also typical for this patient population, with hypertension in particular being the most common co-morbidity observed in several TKA cohorts (Denis et al. 2006 A; Moffet et al. 2004 A; Naylor et al. 2005 A). Additionally, some physiological limitation is qualitatively suggested by the ASA score, again not atypical of TKA recipients (Bozic et al. 2005A; Naylor et al. 2005aA; Pearson et al. 2000 A). Given the self-exertion nature of many rehabilitation interventions, recognising the physiological limitations imposed by concurrent illnesses is an essential consideration in any rehabilitation programme. Likewise, the socioeconomic factors, highlighted as low income and education levels, are associated with poorer pre-operative function (Ackerman et al. 2005 A) and some post-surgical outcomes (Fortin et al. 1999 A). For the therapist, these factors become relevant when setting realistic long-term patient goals and when benchmarking rehabilitation outcomes between surgical units.
REHABILITATION IN THE ACUTE PHASE
OPERATIVE HISTORY AND ACUTE POST-OPERATIVE
PRESENTATION
Relevant operative details:
General anaesthetic + femoral and sciatic nerve blocks.
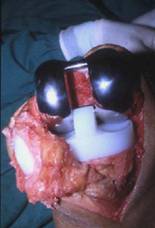
Tri-compartmental primary TKA.
Cemented femoral, tibial, and patella components.
Fixed-bearing, increased congruency, polyethylene bearing.
Posterior cruciate ligament (PCL) sacrificed.
Release of medial collateral ligament.
Anterior cruciate ligament (ACL) removed.
Intra-articular low suction wound drain in situ.
Presentation 18 hrs post-op (Day 1):
Symptoms:
– Reporting 2/10 pain on visual analogue scale, using patient-controlled analgesia c/o numbness and lack of movement in foot.
Mobility:
– In bed, awaiting assessment by physiotherapist.
ROM:
– Start flexion, –10◦.
– End flexion, 60◦.
– Restricted by oedema and crepe bandaging.
– Quadriceps lag, 15◦.
Vital observations:
– BP 110/70 (normally 130/80).
– HR 95–100.
– RR 18.
– SpO2 97 % (3 L/min. O2, nasal prongs).
Blood results:
– Hb 105 g/l.
– Blood glucose level (BGL) 7.7 mmol·l−1.
Other medication:
– Anti-hypertensives and metformin withheld.
– Twice daily protaphane, with top up sliding scale to maintain blood glucose control.3
GENERAL PRINCIPLES
Rehabilitation in the acute phase is largely directed towards the minimisation of the effects of surgical trauma and rendering the patient safe for discharge. The rehabilitative strategies include the use of modalities and techniques to reduce intra- and extra-articular oedema, improve or maintain knee ROM, offset the adverse effects of bed rest, and assist independent ambulation. With respect to the determination of discharge readiness, it is recognised that some surgical units specify a minimum flexion ROM before a patient is deemed fit (Ganz & Benick 2004 Abstract), while others rely more on the level of function achieved (Munin et al. 1998 A; Naylor et al. 2006b A). Though speculative, the latter approach may have evolved secondary to an ever-present need to maintain patient flow in order to keep wait lists in check. In this context, the need to achieve specific physical milestones, such as a minimum flexion requirement, becomes less urgent (Benick et al. 2004 Abstract). It is also recognised that the threshold for discharging patients to an in-patient rehabilitation unit may differ between surgical units, with a lower threshold likely in the private market.
The nature and timing of acute care rehabilitation has also been altered over the last 10 years via the introduction of specific multi-disciplinary care pathways (protocols). Such pathways have procured impressive (up to 50 %) decreases in acute length of stay (LOS) (Brunenberg et al. 2005 A; Dowsey et al. 1998 A; Munin et al. 1998 A; Pearson et al. 2000 A;Wang et al. 1997 A), which must inevitably impact on the goals of rehabilitation, as the therapist-patient interface has contracted considerably at ward level. Finally, central to effective rehabilitation both now and in the longer-term, is good pain management. It is beyond the scope of this chapter to review the evolution of pain management in this context, however; suffice it to say that physiotherapists act as barometers of good pain control in their estimation of whether a patient can engage in their rehabilitation effectively.
3 Additionally, referral to an endocrinologist was initiated on admission, and the recommendation was to add 1/2 80 mg tab of gliclazide twice daily once metformin is recommenced, with the option to increase to 80 mg twice daily if needed (i.e. if HbA1c remains high).
Arthroplasty, knee, Cryotherapy.
Arthroplasty, knee, CPM.
Arthroplasty, knee, walking aid progression.
Arthroplasty, knee, exercises.
Studies were considered appropriate if the subjects had undergone primary TKA, were randomised to receive the treatment(s) under investigation, and the treatment(s) was (were) conducted in the acute in-hospital phase. In cases where a systematic review existed for a given intervention, this predominantly formed the basis for the review, to avoid duplication. Studies focusing on multi-disciplinary and multi-faceted clinical pathways were generally not included. Only studies written in English were reviewed. This review does not include the effects of pre-operative programmes on outcomes. For these, the following reviews are recommended: Ackerman et al. 2004 R; McDonald et al. 2004 R.
QUESTION 1
Does cryotherapy work?
External cooling of the knee surfaces has been shown, in the absence of haemarthrosis, to lower intra-articular temperatures in humans by 2.7–5 ◦C (Martin et al. 2002A).
This, together with the local effects of cold therapy on neural and vascular function, presumably motivates the use of cryotherapy post-TKA for the purposes of reducing pain and swelling. The use of cryotherapy has been observed to be inconsistent in the acute phase following TKA, in terms of both the factors governing its application (Barry et al. 2003) and whether it is utilised at all (Naylor et al. 2005 A, 2006b A).
To date, cryotherapy post-TKA has not been systematically reviewed, but several RCTs have been conducted (Gibbons et al. 2001 A; Healy et al. 1994 A; Ivey et al. 1994 A; Scarcella & Cohn 1995 A; Smith et al. 2002 A; Webb et al. 1998 A). Only one study (Webb et al. 1998 A), comparing cold compression to a non-interventional control, observed significantly less blood transfusions, analgesic consumption, and pain with cold therapy. Of course, the contribution made by the compression component could not be differentiated in this study. Of note, despite the pain relief and blood loss benefits, no differences in ROM acutely or at 12 weeks were observed.
For the majority of the remaining studies in this area, no or minor differences have been observed between those receiving and not receiving early cryotherapy on several outcomes, including LOS, transfusion needs, swelling, ROM, pain, and analgesic use.
Having said this, the interpretation of the impact of cryotherapy in these studies is clouded by comparisons with alternative treatments (such as compression bandaging or alternative cold therapy) (Gibbons et al. 2001 A; Healy et al. 1994 A; Smith et al.2002 A) rather than comparisons with true non-interventional controls Healy and colleagues (1994 A) compared cryotherapy to ice packs. Smith et al. (2002 A) used cold therapy in both groups after 24 hours. Scarcella and Cohn (1995 A), with their sample of 24 TKA patients, were not likely to have had sufficient power to detect differences between their groups when others (Smith et al. 2002 A) have required a sample of 80 for the same outcome variables. Finally, Gibbons et al. (2001 A) did not account for possible gender differences in Hb levels between the treatment and control groups, which themselves differed in their gender profile. This may have explained why cold compression was not associated with a lower transfusion requirement in this study despite being associated with smaller post-operative blood losses. Even with the lack of irrefutable evidence demonstrating that there is no additional benefit from cryotherapy, various authors (Healy et al. 1994 A; Smith et al. 2002 A) have concluded that its costs outweigh its benefits and that compression is preferred in light of this.We conclude that although at this stage it would appear that cryotherapy offers no additional benefits beyond those which could be achieved with compression alone, the methodological limitations of the majority of studies conducted render this issue unresolved.
Regarding Mrs JM, the available evidence does not strongly support or refute the use of cryotherapy, nor is it clear whether compression bandaging alone is superior to it. Thus, the therapist would be justified in trying either. Ideally these modalities would be applied both before and after physiotherapy; at the very least, pain, oedema and ROM should be monitored pre- and post-application. However, Mrs JM’s initial numbness – presumed at this stage to be a hangover from her intra-operative regional anaesthetic – may delay the commencement of ice therapy. Of course, neural deficits beyond 24 hours will need to be differentiated from possible chronic loss due to diabetic neuropathy. Though speculative at this point, the presence of the haemarthrosis following TKA may undermine the impact of external ice applications, rendering the effects of compression bandaging more important.
QUESTION 2
Does continuous passive motion work?
Continuous passive motion (CPM), like cryotherapy, is an adjunctive rehabilitation tool intended to decrease swelling and haemarthrosis, and enhance soft tissue healing and joint ROM (Milne et al. 2003 A). In contrast to cryotherapy, however, CPM has been subject to many RCTS involving TKA recipients (n=59), one Cochrane review (Milne et al. 2003 A), and one qualitative review (Lachiewicz 2000 R). Thus, more definitive conclusions can be drawn regarding its effectiveness.
Milne et al. (2003 A), based on a meta-analysis, concluded that CPM combined with standard physiotherapy was associated with a small increase in flexion ROM at two weeks (4.3◦ weighted mean difference (WMD4)), decreased LOS (0.69 days WMD),and a decreased risk of manipulation within the first month (relative risk 0.12).
4 WMD: difference between control and treatment group is weighted by the inverse of the variantion.
CPM was not found to improve passive ROM. The authors did conclude, however, that information and protocol biases were present in the review due to inadequate reporting of some variables (for example, whether ROM was passive or active) and inconsistent protocols (for example, pain relief and pre-operative education) across studies. Information on ideal dose and application could not be derived. In light of these facts, the authors recommended that the potential benefits of CPM be weighed against the possible increased costs and inconvenience, and that more research be conducted to determine the optimum treatment parameters. Not included in the analyses were the effects of CPM on midline wound healing, bleeding overall, and hospital costs. These have been shown to be a concern in some trials (Lachiewicz 2000 R).
Since the publication of the meta-analysis by Milne and colleagues, only one other RCT has been conducted in TKA patients. Denis et al. (2006 A) did not observe any differences in discharge (∼ eight days post) ROM, LOS,WOMAC function, and TUG times between those treated with conventional physiotherapy plus 35 or 120 minutes of CPM daily, and those receiving conventional physiotherapy only.With the exception of LOS, these results confirm the conclusions of the aforementioned metaanalysis.
It is unfortunate, however, that the number of manipulations post-discharge was not monitored given that this is perhaps the most clinically relevant outcome concerning CPM. In terms of current clinical practice, we observed that CPM does not appear to be in routine use in Australia (Naylor et al. 2006b A). Whether this is the case elsewhere is unknown as there are no other survey data concerning this. We also observed in our unit, where CPM was routinely prescribed, that only 40 % of patients received it (Naylor et al. 2005 A). Protocol deviance was explained by a combination of lack of awareness of the protocol by rotating physiotherapists, and their perceived lack of need – the latter possibly explained by the fact that functionality and not ROM primarily determines discharge at our unit. At this point in time, our CPM practices, together with our pain relief and pre-operative education policies, are under review, as the number of manipulations performed within six months of surgery has increased in recent times.
Regarding Mrs JM, in view of the risk of manipulation alone, CPM should be initiated at least once per day for several hours during bed rest periods. This recommendation ideally applies to units where CPM is readily available and where medical and nursing staff can apply it. Though speculative, CPM may be of particular benefit to Mrs JM given her poorly controlled diabetes (evidenced by the elevated HbA1c of 8.2 %; non-diabetic range 3–6 %). Glycosylation (permanent protein modification by glucose) of collagen or elastin as a result of persistently high BGL may result in tissue stiffness (Paul & Bailey 1996 B), hence Mrs JM may be at a greater risk of manipulation.5
5 22%of patients presenting for manipulation under anaesthesia for frozen shoulders had diabetes (Hamdan
& Al-Essa 2003).
QUESTION 3
What is the evidence for exercise and early ambulation to improve ROM, decrease length of stay, and prevent deep venous thrombosis?
Only one study has compared the outcomes of patients who received formal knee flexion exercises in addition to standardised physiotherapy with those who received standardised physiotherapy only (Ganz & Ranawat 2004 Abstract). Though formal knee flexion exercises were associated with greater active knee flexion at one week, this did not translate into any functional differences (such as stair ambulation or use of aids) or shorter LOS. At three and 12 months, there were no differences in active knee flexion. No studies were found focusing on active knee extension. Despite the lack of evidence in support of specific active exercises, we have observed the prescription of lower limb exercises in the acute stage to be routine in Australia (Naylor et al. 2006b A). This notwithstanding, as there does not appear to be any routine case to suggest active exercises are detrimental in this patient group, we find no reason for not including them in the therapy repertoire.
Similarly to active exercises, the arguments for early ambulation post-TKA rest largely on the desire to minimise the well-known adverse effects of bed rest and to accelerate discharge from hospital. To our knowledge, only one RCT has been conducted (Munin et al. 1998 A) which highlights the specific benefits of early rehabilitation, including early ambulation (commencing Day Three versus Day Seven), on LOS, functional performance, and Deep Vein Thrombosis rate. Though the specific contribution attributable to early ambulation alone cannot be reliably estimated, the absence of evidence to the contrary suggests protocols aimed at early ambulation are desirable. We do qualify this statement, however, in that we recommend an assessment of the patient’s medical stability (including blood pressure, heart rate and rhythm, BGL, oxygen saturation levels, and Hb) precedes any physiotherapy intervention.
Regarding Mrs JM, her lower limb neural deficit will preclude ambulation and some bed exercises until it resolves. A combination of closed- and open-chain isometric, concentric, and eccentric exercises will be prescribed for the flexor and extensor muscle groups in her lower limbs. Ambulation will commence after removal of the wound drains. Her cardiovascular history necessitates close monitoring of her vital signs prior to her participating in any exercise, however. Her low Hb is typical at this stage, given the acute blood losses (mean 608 mls) associated with the surgery (Naylor et al. 2005 A), and, at her current level, does not warrant a transfusion (NH&MRC & ASBT 2001 A).
QUESTION 4
What evidence guides walking aid progression?
The literature search yielded no RCTs investigating the optimal rate of walking aid progression. We are aware of surgical units that dictate the rate of progression according to the presence or absence of cement. In our unit, all patients are progressed and discharged on crutches, with instructions to weight-bear as tolerated unless otherwise indicated. It is not clear at this stage whether the rate of progression onto a walking stick or to complete independence from walking aids is a concern for longterm prosthesis stability, the restoration of normal gait patterns, or the evolution of back pain.
QUESTION 5
Does electrical stimulation work?
The electrical stimulation of the knee extensor muscles post-TKA is based on the premise that voluntary activation is not sufficient to restore strength (Avramidis et al. 2003A). Three studies were identified that randomised the use of electrical stimulation to the vastus medialis or quadriceps femoris during CPM, commencing in the acute period and given alongside a standardised physiotherapy programme. Gotlin et al. (1994 A) and Haug and Wood (1988 A) observed that patients receiving two to three hours of muscle stimulation daily until discharge experienced less extensor lag and shorter LOS. In a longer-term study, Avramidis et al. (2003 A) observed that patients receiving electrical stimulation for two hours twice daily from the second post-operative day for six weeks, attained a faster walking speed at six weeks, and this effect carried over until the 12th week. The authors concluded that the greater walk speed was a consequence of more rapid quadriceps recovery and, as such, a greater ability to participate in exercise. It should be noted that the control group did not receive any standardised physiotherapy post-discharge. The addition of a third group that received standardised physiotherapy for six weeks would have helped to clarify whether electrical stimulation was superior to or simply a replacement for voluntary muscle activation. While the use of electrical stimulation looks promising, the technical and potentially cumbersome nature of the procedure, and the prerequisite for effective communication between patient and therapist for safety reasons, may have deterred widespread adoption of this treatment option.
Regarding Mrs JM, assuming availability of the device and competency of both the staff and patient in its use, intermittent neuromuscular stimulation is an appropriate rehabilitation intervention, given her quadriceps lag.
QUESTION 6
What is the evidence for hydrotherapy?
No RCTs were identified concerning the efficacy of hydrotherapy post-TKA. We recognise that it is a treatment option where facilities exist (Naylor et al. 2006b A) and that a non-randomised trial has been conducted in Germany (Erler et al. 2001 A).
No recommendations can be made at this stage, but note that, at the very least, the integrity of thewound is paramount for hydrotherapy to be considered a viable option. REHABILITATION IN THE POST-DISCHARGE PHASE
GENERAL PRINCIPLES
The post-discharge phase of rehabilitation commences after discharge from an acute care facility. Goals of rehabilitation in the earlier post-discharge phase focus upon increasing the level of independence of the patient, which may include weaning off a walking aid, maintaining and improving knee joint ROM, controlling or reducing residual oedema, increasing muscle strength and endurance, and gradual return to work and leisure activities. In the later phase of post-discharge rehabilitation, goals include further improvement of muscle strength and endurance, improvement of cardiovascular fitness, and full return to work and leisure activities.
The sources of evidence reviewed for specific rehabilitative interventions in the post-acute phase consisted of RCTs and systematic reviews. In order to identify the relevant literature, the following combinations of terms were used in an electronic literature search of MEDLINE, CINAHL and EMBASE:
Total knee replacement, with subject headings: arthroplasty, replacement, knee; knee prosthesis; TKR. Rehabilitation, with all subject headings. Physiotherapy, with subject headings: exercise therapy; orthopedics; physical therapy (specialty); physiotherapy. The initial literature search yielded 230 studies. For the present review, studies were only included if the subjects had undergone primary TKA, were randomised to receive the treatment(s) under investigation, and the treatment(s) was (were) conducted in the post-acute phase. Only studies written in English were reviewed. Only five trials satisfied these criteria, thus revealing the paucity of evidence for effects of rehabilitation in the post-acute phase. One study (Mitchell et al. 2005 A) included pre-operative physiotherapy in one group and was thus excluded. The remaining four trials differed markedly in their methodology and investigated the effects of out-patient physiotherapy versus home-based rehabilitation (Kramer et al. 2003 A; Rajan et al. 2004 A); traditional versus functional home-based exercise (Frost et al. 2002 A); and intensive versus usual care treatment (Moffet et al. 2004 A). Due to the limited number of studies identified and the holistic nature of the physiotherapy programmes described, it was not possible to examine the effect of a single treatment component in the postacute phase. In addition to the five reports of randomised trials, one recent review that presented current evidence from experts on knee and hip arthroplasty was identified (Jones et al. 2005 R).
QUESTION 7
What is the evidence supporting early post-discharge rehabilitation?
Three RCTs have examined the effects of physiotherapy provided in the early postdischarge phase of rehabilitation; that is, commencing immediately after discharge from acute care. Kramer et al. (2003 A) investigated effects of clinic- versus homebased rehabilitation. All patients were provided with advice on knee management and were prescribed home strengthening and ROMexercises, the basic form of which they were taught during the acute in-patient period. The home-based group received weekly phone calls from a physiotherapist, whereas patients in the clinic-based group attended the clinic once or twice weekly until three months post-operation. At three and 12 months post-operation there was no difference between groups on any outcome measures, which included WOMAC total and pain and function subscales, SF-36 total, knee flexion range, 30-second stair test, and 6MWT. Similarly, another study (Rajan et al. 2004 A) found no additional benefit of out-patient physiotherapy compared with a home exercise programme at three, six, or 12 months; however, there was no description of the physiotherapy interventions, and the only outcome measure reported was knee flexion range. Provided that sufficient knee range is available for performance of ADL, this outcome measure is a poor sole criterion upon which to judge treatment efficacy in the post-acute phase. Frost et al. (2002 A) compared two home-based programmes – usual care (for example, ROM exercises, quadriceps, and hamstrings strengthening) versus functional exercises (rising from a chair, lifting the leg onto a step, and walking) – that commenced immediately after hospital discharge. At the one-year follow-up assessment there was no difference between groups in 10 m walking speed, pain, knee flexion range, or leg extensor power.
All three of the above studies used intention-to-treat analysis; one study employed therapist blinding (Frost et al. 2002A) and another, partial blinding (Kramer et al. 2003 A), and subjects were randomly allocated to groups. Losses to follow-up were 3 % (Rajan et al. 2004A), 23%(Kramer et al. 2003A), and 43%(Frost et al. 2002A), and all studies described reasons for drop-out. Very few adverse events occurred using the exercises prescribed in these studies. According to the principles of evidence-based practice (Herbert et al. 2005 A/R), the Physiotherapy Evidence Database (PEDro) assigned the following scores to each of the studies: Frost et al. 6/10; Kramer et al. 6/10; and Rajan et al. 7/10; indicating that these studies all provide a moderate level of evidence. It can be concluded that patient outcomes one year post-TKA are not affected by location of rehabilitation delivery (out-patient physiotherapy clinic versus home) or type of exercise (usual versus functional). However, loss to follow-up may be affected by the level of supervision provided by the physiotherapist (out-patient attendance or phone call monitoring versus no monitoring). Larger trials, which provide a greater power to detect small differences in outcome measures, may necessitate revision of these conclusions. Patient outcomes at one year post-TKA indicate that although significant improvements were evident compared to before surgery, there is still a residual level of pain, disability, and loss of knee flexion range; and that patients only just attain the lower limits of age-matched normal function, for example walking speed. A lack of sufficient exercise intensity during rehabilitation may partly contribute to these shortfalls in recovery, but it was not possible to calculate exercise dosage from these trials since exercise intensity was largely patient determined or else it was not described.
QUESTION 8
What is the evidence supporting later post-discharge rehabilitation?
One RCT only (Moffet et al. 2004 A) has examined the effect of commencing rehabilitation later in the post-discharge phase. Ability to exercise in this stage would be anticipated to be greater than in the early post-acute phase, when anaemia, pain, oedema, and residual effects of anaesthesia can cause significant limitation. Moffet et al. (2004 A) employed intention-to-treat analysis: blinding of evaluators; random allocation of subjects; and had only ∼10 % loss to follow-up, with all drop-outs being described, thus providing a moderate to strong level of evidence (PEDro score 7/10). Two months after TKA, patients were randomised to either usual care (strength training, ROM exercise, ice, gait retraining; 26 % also received home visits) or to an intensive 12-week supervised physiotherapy programme, which also included the usual care components. Intensive sessions included strength (for example, maximal isometric contractions of quadriceps and hamstrings; functional exercises such as sit-to-stand and stairs) and endurance exercise training (walking or cycling at 60– 80 % of maximum predicted heart rate for up to 20 min.). Exercise intensity was progressed as required, however, while number of repetitions was reported, intensity of strength training was difficult to assess from the data provided. No adverse events from treatment occurred. At six months post-TKA, patients in the intensive exercise group had increased their 6MWT by 31 % (93 m), compared to 25 % (72 m) increase in the usual care group; a significant effect size between interventions of ∼9 %. Significant treatment effect differences of a similar magnitude were evident in the WOMAC subscales of pain, stiffness, and difficulty in performing ADL. One year after TKA, patients in the intensive group tended to have a higher 6 min. Walk Test distance (P = 0.06; 400 m or ∼1.1 m·s−1, which placed them at the lower limit of normal for their age) than the control group (370 m; 1.03 m·s−1), and both groups had similar levels of pain, stiffness, and difficulty performing tasks. This study demonstrates that more intensive rehabilitation, commenced in the later post-acute phase, results in greater improvements in walking speed at six months post-TKA (and probably also at 12 months, given the near statistical significance and relatively low subject number). Therefore, usual care physiotherapy after TKA probably provides less than optimal stimuli, and patients could likely make further significant gains if sufficiently challenged in the post-discharge rehabilitation period. Further, the authors suggest that increasing the exercise intensity and prolonging the programme may yield greater treatment effects. If so, this not only has important functional relevance for the patient, but also has implications for the progression or retardation of common co-morbidities such as hypertension and type 2 diabetes.
POST-DISCHARGE REHABILITATION FOR MRS JM
Mrs JM has similar co-morbidities (HT, diabetes, cardiac disease) and is of a similar age to the patients in the Moffet et al. (2004 A) study. Her scores for each of the WOMAC subscales are two- to three-fold higher than those reported at two months post-TKA, and are anticipated to improve considerably after surgery. Ideally, Mrs JM’s early post-acute rehabilitation will be conducted from home; however, a retrospective review of effects of the co-morbidities of HT, diabetes, and obesity (all of which Mrs JM suffers from) in 959,839 patients after arthroplasty found that each of the co-morbidities was an independent predictor of increased post-operative complications and non-homebound discharge (Jain et al. 2005A). Additionally, achievement of rehabilitation goals by Mrs JM may be slowed by the presence of OA in her left knee (unoperated). For example, progression from a walking aid to independent ambulation, or the recovery and improvement of walk speed, may be delayed by poor ipsilateral or contralateral joint dysfunction. Certainly, with respect to the latter, our data demonstrate that 15-m walk and TUG times are slower in patients with a knee or hip replacement awaiting further surgery for other joints than in patients with knee or hip replacement who are not (Naylor et al. 2006a A).
Based on the evidence from theRCTsdiscussed above, in the early post-acute phase, Mrs JM will be prescribed an exercise programme that includes ROM and strength exercises (including functional exercise), and gait retraining; and she will receive advice regarding management of oedema and pain. Mrs JM will remain relatively anaemic (Hb 105 g·l−1) at discharge, which may result in mild fatigue, dizziness, and dyspnoea during more demanding submaximal exercise, as a consequence of lower arterial oxygen content. This, coupled with pain, oedema, and the associated muscle inhibition, will reduce the exercise intensity that Mrs JM can undertake in this early period. In addition, given Mrs JM had poor pre-operative control of her diabetes (indicated by the HbA1c), she may experience more difficulty controlling her BGL in the post-acute phase consequent to reduced activity, stress, and hospitalisation. Even so, current opinion (Sigal et al. 2004 A) is that light- or moderate-intensity exercise should not be postponed in those with type 2 diabetes, even if BGL exceeds ∼17 mmol·l−1 (300 mg·dl−1), unless the patient feels unwell and has urinary or blood ketones. We anticipate improved blood glucose control in this case, following the review by the endocrinologist in hospital and the consequent addition of gliclazide to Mrs JM’s usual metformin. Advice from a diabetes educator and a dietician will also enhance her management. Her programme can be conducted at home, with a weekly phone call from her physiotherapist to assess her ability to complete the exercises, to advise on exercise progression, and to monitor potential complications. In the later post-discharge phase, Mrs JM will attend out-patient physiotherapy for a more intensive programme. The programme will commence once oedema and pain have subsided; probably about six to eight weeks post-surgery, and will build upon the gains made with therapy in the acute period. Additionally, based on our recent audit of acute and short-term outcomes following TKA (Naylor et al. 2005 A), we anticipate that Mrs JM will have recovered to ∼90 % of her pre-operative Hb (∼125 g·l−1) by about the sixth week post-TKA. Given the presence of type 2 diabetes mellitus, hypertension, and IHD, current recommendation (Sigal et al. 2004 A) is that it would be prudent to have Mrs JM formally assessed for cardiovascular risk prior to commencing more intense exercise (if not done comprehensively pre-operatively).
Following individual evaluation and exercise prescription, her programme can be undertaken in a group setting, which may be a more cost-effectiveway to deliver more intense, supervised rehabilitation, and may enhance motivation. The programme will include lower limb strength training, functional exercises to promote strength and balance, stretches, and either cycling or walking for local muscular and whole-body endurance. Intensity will be monitored by heart rate and rating of perceived exertion (the latter particularly, if any autonomic neuropathy is suspected or demonstrated), and the number of repetitions and sets of each strength exercise, and the load and duration of endurance exercises will be recorded. As previously stated, the current evidence does not provide sufficient detail to determine exercise dosage for resistance training; hence the following suggestions are based upon research drawn from other sources, and are subject to change when further specific evidence regarding resistance training afterTKAis published. The intensity of resistance exercise will be gradually increased as tolerated, beginning with one set of 10–15 repetitions (not to fatigue) twice per week, and over a number of weeks progressing to three sets of eight repetitions at a 10 RM(repetition maximum) load up to three times per week. The latter is recommended for individuals with type 2 diabetes, to assist with improving metabolic control, for example lowering HbA1c (Sigal et al. 2004 A) – a very desirable outcome in Mrs JM. Resistance exercise is also recommended for patients with OA; however, it is suggested that muscles should not be exercised to fatigue (American Geriatrics Society Panel on Exercise and Osteoarthritis 2001 A). Hence, resistance training for the left leg (knee OA) will be conducted at a lower load and not to fatigue (for example, eight to 10 repetitions at 15 RM load) and will be changed to isometric exercise if the left knee becomes unstable or acutely inflamed. Endurance exercise (walking or cycling) will be commenced at 50 % of maximum heart rate for five to 10 minutes at least every second day, and progressed as tolerated to a weekly dose of 150 minutes at 50–70 % of maximum heart rate (Sigal et al. 2004 A). Based on the results of Moffet et al. (2004 A), Mrs JM can expect to be walking ∼30 % further in a 6MWT after six months; perhaps even more quickly if she has a more intense exercise programme that is continued for a longer period (depending on the degree of limitation from her left knee OA). The ability to undertake both sustained aerobic and resistance exercise is important in addressing Mrs JM’s co-morbidities of obesity, type 2 diabetes, HT, and IHD, and in accomplishing a full return to her ADL (including negotiation of 18 stairs at home) and leisure activities (lawn bowls). In addition, consultation with a diabetes educator and a dietician are recommended for Mrs JM.
Thus it appears that most rehabilitation programmes finish just when the patient is becoming more capable of exercising with greater intensity. The incorporation of more challenging (more intense) exercise may address the deficits in gait speed, muscle strength, and quality of life evident several months to years after TKA (see Introduction).
Given the common occurrence of co-morbidities in patients who undergo TKA, a more protracted exercise programme, which included both strength and endurance components, would be anticipated to have important health and financial benefits.
However, given the paucity of RCTs and the holistic nature of the existing post-acute physiotherapy RCTs, there is little evidence to suggest what the optimal exercise programme after TKA might comprise.
IMPACT OF SURGICAL FACTORS ON LONGER TERM RECOVERY
QUESTION 9
Do prosthesis design and surgical choice impact on rehabilitation or functional recovery? Despite myriad investigations concerning efficacy of TKA, there is significant variation in the prostheses used and surgical decisions made. In other words, despite substantial evidence supporting the intervention, best surgical practice in this field is yet to be recognised. The need to re-align the knee to a neutral mechanical axis and balance the soft tissue is generally agreed upon; some of the issues that remaid debated in the literature include cemented versus uncemented implants, the role of the posterior cruciate ligament, mobile versus fixed bearing, and whether or not to resurface the patella.
Cemented versus uncemented fixation
Mrs JM underwent a cemented TKA. Cemented TKA remains the standard to which alternative forms of fixation need to be compared (Insall et al. 1976 A; Jones et al. 2005 A; Rodriguez et al. 2001 A). In Australia, cemented TKA make up almost 50 % of procedures, while uncemented and hybrid implants comprise 25 % each (Australian Orthopaedic Association National Joint Replacement Registry 2004). Uncemented fixation has the theoretical advantage of osseointegration, which may have implications for longevity, infection, and future bone loss (Diduch et al. 1997 A), while cemented implants have a significant cost benefit. In general terms, failure of uncemented implants has been mainly on the tibial and patella surfaces. Many early designs showed pain scores that were slower to improve than in their cemented counterparts, and had higher revision rates (Duffy et al. 1998A; Ritter 2001A). Newer implant designs may have overcome these problems; however, long-term results are yet to be realised. To date there is no literature examining the impact of weight bearing on early and late fixation in cemented or uncemented prostheses.
Cruciate versus no cruciate
While most current prostheses sacrifice the ACL, controversy remains regarding the PCL. Some argue that preservation of the PCL aids in improving the stability, kinematics, and mechanics of the knee replacement and avoids extra bone resection (Rand 1996 A). Those in favour of excision argue that the PCL is not normal in arthritic knees and that its excision allows improved balancing and correction of deformity (Hirsch et al. 1994 A), as well as more consistent and predictable kinematics (Dennis et al. 1996 A; Dennis et al. 1998 A). Excellent clinical results have been shown with both PCL-retaining and -sacrificing TKAs. Nevertheless, there remain significant differences in the kinematics between normal and replaced knees, and much of this gait abnormality is thought to be related to cruciate deficiency. This, combined with senile muscle weakness and prolonged disability, may further reduce the ability of patients to perform activities, including rehabilitative activities, following TKA.
Two options are available to improve stability and kinematics following resection of the ACL, PCL, or both. One option is to increase the congruity of the polyethylene with anterior and posterior lips, to prevent translation of the tibia relative to the femur.
The other option is for the surgeon to introduce a cam-and-post mechanism, which prevents posterior translation of the tibia relative to the femur. Mrs JM had a posterior stabilised cam-and-post type implant. It is important to recognise that neither PCL retaining nor -substituting implants provide varus or valgus stability, and they both require intact collaterals for stability.
A recent RCT (Straw et al. 2003 A) examined the effect of the PCL in total knee arthroplasty. Patients were randomised to retention or excision of the PCL. There were four groups: (a) PCL retaining and standard implants; (b) PCL released and standard implants; (c) PCL excised and standard implants; (d) PCL excised and posterior substituting implants. There was no difference in groups (a), (c) and (d) with regards to pain scores, range of motion, knee scores, or functional scores. Patients in group (b), with retaining implants and a released PCL, did significantly worse than the other three groups in terms of knee scores and function. The posterior stabilised group (d) had the highest functional scores, walking distance, and stair climbing. The poorest range of motion was in group (a), suggesting tightness in flexion with PCL retention. In terms of clinical stability, posterior stabilised (d) were the most stable, while the excised group (c) were the most lax in the anteroposterior plane; this was not statistically significant, however. There was no difference between groups in terms of mediolateral stability. Follow-up averaged 3.5 years and as such the issue of long-term wear could not be examined.
Integrity of the collateral ligaments
Release of the collateral structures is required during TKA when the gaps created for the implants in flexion and extension are not rectangular. If left asymmetrical, this can lead to asymmetric forces on the medial or lateral sides of the knee and potentially cause pain, instability, poor function and early wear. Creating equal gaps requires correct bony alignment as well as appropriate release of the soft tissues. In the varus knee, medial structures tend to be tight, while in the valgus knee, it is the lateral structures that become tight. The contributing structures will depend on whether the knee is tight in an extended or flexed position. On the medial aspect, the medial collateral ligament and postero-medial capsule may require releasing to balance the knee (Whiteside 1995A). On the lateral aspect, the lateral collateral, popliteal tendon, iliotibial band and capsule may need releasing for balance (Whiteside 1999 A). Mrs JMrequired release of the medial collateral ligament to balance the knee. Occasionally in severe deformity, the opposite side attenuates (for example, medial structures in a valgus knee), thus, requires attention. If so, surgical reconstruction of the ligament is performed, a more constrained form of implant is used, or both. Instability following collateral release does not occur provided that the mechanical axis of the leg has been corrected with the surgery. Ligament releases still leave peripheral attachments and other soft tissue connections, such as periosteum or capsular tissue, which allow the released ligaments to function (Whiteside 2005 A). Ongoing clinical instability, perhaps detected by the therapist if not reported by the patient, usually occurs in the presence of overall limb malalignment, inadequate soft tissue release, or inadvertent transection of ligamentous structures. By and large, collateral release should not impede functional recovery or rehabilitation.
Fixed versus mobile bearing implants
While fixed bearing implants yield excellent results, mobile bearing prostheses were introduced to try and improve wear characteristics, range of motion, and longevity. These implants have dual articulation, with a highly conforming articular surface between the femur and the polyethylene insert. Many designs exist and they vary in the degree of movement allowed between the polyethylene and the base plate. One long-term non-randomised study reported similar clinical and prosthesis survivorship results to fixed bearing implants (Buechel 2002 A), but the impact of activity per se, either early or late, was not addressed. It is tempting to speculate that, given the equivalent prosthesis survivorship across the two designs, neither activity level nor type of activity impacts on long-term functional recovery. However, non-randomised allocation of patients to the varying prosthetic designs may contribute to this; thus, RCTs are ideally needed to confirm this notion. It is also worth noting that trials subjecting the same prostheses to differing long-term in vivo mechanical loading (such as functional and exercise loads) have not been conducted.
Patella resurfacing versus non-resurfacing
Controversy remains over whether or not to resurface the patella. Mrs JM had a cemented patella resurfacing. Many of the early problems with the patellofemoral joint have been addressed by improving the characteristics of the femoral component (Andriacchi et al. 1997 A) and, as such, much of the older literature may not be relevant today. Ongoing anterior knee pain is the reason for considering resurfacing, while complications including patella fracture, extensor mechanism disruption, and loosening are reasons to avoid this option routinely. Resurfacing of the patella is generally agreed upon in inflammatory arthritis, patella maltracking, eburnated bone on the patella, preoperative anterior knee pain, and crystalline deposition disease (Kajino et al. 1997A; Kim et al. 1999A). Studies in patients with bilateral arthroplasty with only one side resurfaced have not shown significant differences (Keblish et al. 1994 A). While there is equivocal evidence from RCTs (Barrack et al. 2001 A;Wood et al. 2002 A), a recent review (Holt & Dennis 2003 R) concluded that, although patient selection is critical to the decision to resurface the patella, unresurfaced patellae deteriorate over time and secondary resurfacing is associated with greater residual patellofemoral pain. This was reiterated by Jones et al. (2005 R), who also concluded that patella resurfacing is likely to improve outcomes, including long-term pain-free patella function. From the therapist’s perspective, knowledge of whether or not the patella was resurfaced may help explain ongoing or residual anterior knee pain, or even pain emerging within a few months to years of the TKA procedure. To our knowledge, there are no context-specific data available to guide the therapist in terms of what, if any, lower limb exercises are preferred in the presence or absence of patella resurfacing.
SUMMARY
The choices that surgeons face when undertaking TKA are manifold. Unfortunately, well constructed RCTs are not available to answer many of the debates that remain, particularly in relation to TKAs’ relevance to rehabilitation. However, from a surgeon’s perspective, there is little doubt that good alignment and good balance are the most important features in providing patients with a well-performing, long-lasting joint replacement. Provided these principles are adhered to, and once best practice rehabilitation is identified, we assume at this stage that post-operative physiotherapy and rehabilitation should not be substantially affected by variations in surgical hardware and technique. Having said that, note that the more cognisant the physiotherapist is of each patient’s surgical particulars, the less risk there is of their doing harm, and the better placed they are to set pragmatic rehabilitation goals.
REFERENCES
Aarons H, Hall G, Hughes S, Salmon P (1996) Short-term recovery from hip and knee arthroplasty.
Journal of Bone and Joint Surgery 78B: 555–558.
Ackerman IN, Bennell KL (2004) Does pre-operative physiotherapy improve outcomes from lower limb joint replacement surgery? A systematic review. Australian Journal of Physiotherapy 50: 25–30.
Ackerman IN, Graves SE, Wicks IP, Bennell KL, Osborne RH (2005) Severely compromised quality of life in women and those of lower socioeconomic status waiting for joint replacement surgery. Arthritis & Rheumatism 53: 653–658.
American Geriatrics Society Panel on Exercise and Osteoarthritis (2001) Exercise prescription for older adults with osteoarthritis pain: consensus practice recommendations. Journal of the American Geriatrics Society 49: 808–823.
Andriacchi TP, Yoder D, Conley A et al. (1997) Patellofemoral design influences function following total knee arthroplasty. Journal of Arthoplasty 12: 243.
Australian Orthopaedic Association National Joint Replacement Registry (2004) Annual Report Adelaide: Australian Orthopaedic Association.
Avramidis K, Strike PW, Taylor PN, Swain ID (2003) Effectiveness of electric stimulation of the vastus medialis muscle in the rehabilitation of patients after total knee arthroplasty.
Archives of Physical Medicine and Rehabilitation 84: 1850–1853.Bachmeier CJM, March L, Cross M,
Lapsely H, Tribe K, Courtenay B et al. (2001) A comparisonof outcomes in osteoarthritis patients undergoing total hip and knee replacement surgery. Osteoarthritis and Cartilage 9: 137–146.
Barrack RL, Bertot AJ, Wolfe MW, Waldman DA, Milicic M, Myers L (2001) Patellar resurfacing
in total knee arthroplasty: a prospective, randomized, double-blinded study with five to seven years of follow-up. Journal of Bone and Joint Surgery 83A: 1376–1381.
Barry S, Wallace L, Lamb S (2003) Cryotherapy after total knee replacement: a survey of current practice. Physiotherapy Research International 8: 111–120.
Bellamy N, BuchananW, Goldsmith C, Campbell J, StittL(1988)Validation study ofWOMAC:
a health status instrument for measuring clinically-important patient-relevant outcomes
following total hip or knee arthroplasty in osteoarthritis. Journal of Orthopaedic Rheumatology
1: 95–108.
Benedetti MG, Catani F, Bilotta TW, Marcacci M, Mariani E, Giannini S (2003) Muscle activation pattern and gait biomechanics after total knee replacement. Clinical Biomechanics 18: 871–876.
Benick RA, Backus SI, Kroll MA, Ganz SB, MacKenzie CR (2004) Knee flexion and functional ambulatory status following unilateral total knee arthroplasty. Topics in Geriatric Rehabilitation 20: 308.
Berth A, Urbach D, Awiszus F (2002) Improvement of voluntary quadriceps muscle activation after total knee arthroplasty. Archives of Physical Medicine and Rehabilitation 83: 1432– 1436.
Bischoff HA, Roos EM (2003) Effectiveness and safety of strengthening, aerobic, and coordination exercises for patients with osteoarthritis. Current Opinion in Rheumatology 15: 141–144.
Bozic KJ, Durbhakula S, Berry DJ, Naessens JM, Rappaport K, Cisternas M et al. (2005) Differences in patient and procedure characteristics and hospital resource use in primary and revision total joint arthroplasty. Journal of Arthroplasty 20: 17–25.
Brosseau L, Davis J, Drouin H, Milne S, Noel M, Robinson VA et al. (2006) Continuous passive motion following total knee arthroplasty. Cochrane Library 1 http:// www.thecochranelibrary.comCD004260.
Brunenberg DE, van Steyn MJ, Sluimer JC, Bekebrede LL, Bulstra SK, Joore MA (2005) Joint recovery programme versus usual care: an economic evaluation of a clinical pathway for joint replacement surgery. Medical Care 43: 1018–1026.
Buechel FF Sr (2002) Long-term follow-up after mobile-bearing total knee replacement. Clinical Orthopaedics and Related Research 404: 40–50.
Denis M, Moffet H, Caron F, Ouellet D, Paquet J, Nolet L (2006) Effectiveness of continuous passive motion and conventional physical therapy after total knee arthroplasty: a randomized clinical trial. Physical Therapy 86: 174–185.
Dennis DA, Komistek RD, Hoff WA, Gabriel SM (1996) In vivo knee kinematics derived using an inverse perspective technique. Clinical Orthopaedics and Related Research 331: 107–117.
Dennis DA, Komistek RD, Colwell CE Jr, Ranawat CS, Scott RD, Thornhill TS et al. (1998) In vivo anteroposterior femorotibial translation of total knee arthroplasty: a multicenter analysis. Clinical Orthopaedics and Related Research 356: 47– 57.
Diduch DR, Insall JN, Scott WN, Font-Rodriguez D (1997) Total knee replacement in young, active patients: long-term follow-up and functional outcome. Journal of Bone and Joint Surgery 79A: 575–582.
Dixon T, Shaw M, Ebrahim S, Dieppe P (2004) Trends in hip and knee joint replacement: socioeconomic inequalities and projections of need. Annals of Rheumatic Diseases 63: 825–830.
Dowsey M, Kilgour ML, Santamaria NM, Choong FM (1998) Clinical pathways in hip and knee arthroplasty: a prospective randomized controlled study. Medical Journal ofAustralia
170: 59–62.
Duffy GP, Berry DJ, Rand JA (1998) Cement versus cementless fixation in total knee arthroplasty.
Clinical Orthopaedics and Related Research 356: 66–72.
Ethgen O, Bruyere O, Richy F, Dardennes C, Reginster JY (2004) Health-related quality of life in total hip and total knee arthroplasty. Journal of Bone and Joint Surgery 86A: 963– 971.
Erler K, Anders C, Fehlberg G, Neumann U, Brucker L, Scholle HC (2001) Measurements of results of a special hydrotherapy during in-patient rehabilitation after implantation of a total knee arthroplasty. Zeitschrift f¨ur Orthop¨adie und ihre Grenzgebiete 139: 352–358.
Fortin PR, Penrod JR, Clarke AE, St-Pierre Y, Joseph L, Belisle P et al. (2002) Timing of total joint replacement affects clinical outcomes among patients with osteoarthritis of the hip or knee. Arthritis & Rheumatism 46: 3327–3330.
Fortin PR, Clarke AE, Liang JL, Tanzer M, Ferland D et al. (1999) Outcomes of total hip and knee replacement: preoperative functional status predicts outcomes at six months after surgery. Arthritis & Rheumatism 42: 1722–1728.
Fransen M, Crosbie J, Edmonds J (2003) Isometric muscle force measurement for clinicians treating patients with osteoarthritis of the knee. Arthritis & Rheumatism 49: 29–35.
Fransen M, Crosbie J, Edmonds J (2001) Physical therapy is effective for patients with osteoarthritis of the knee: a randomized controlled clinical trial. Journal of Rheumatology 28: 156–164.
Fransen M, McConnell S, BellM(2002) Therapeutic exercise for people with osteoarthritis of the hip or knee: a systematic review. Journal of Rheumatology 29: 1737–1745.
Frost H, Lamb SE, Robertson S (2002)Arandomized controlled trial of exercise to improve mobility
and function after elective knee arthroplasty. Feasibility, results and methodological difficulties. Clinical Rehabilitation 16: 200–209.
Ganz SB, Benick RA (2004) A comparison of functional recovery following unilateral and bilateral total knee arthroplasty. Topics in Geriatric Medicine 20: 310.
Ganz SB, Ranawat CS (2004) Efficacy of formal knee flexion exercises on the achievement of functional milestones following total knee arthroplasty. Topics in Geriatric Medicine 20: 311.
Gibbons CER, Solan MC, RickettsDM,PattersonM(2001) Cryotherapy compared with Robert Jones bandage after total knee replacement: a prospective randomized trial. International Orthopaedics 25: 250–252.
Gotlin RS, Hershkowitz S, Juris PM, Gonzalez EG, Scott WN, Insall JN (1994) Electrical
stimulation effect on extensor lag and length of hospital stay after knee arthroplasty.
Archives of Physical Medicine and Rehabilitation 75: 957–959.
Gur H, Cakin N, Akova B, Okay E, Kucukoglu S (2002) Concentric versus combined
concentric-eccentric isokinetic training: effects on functional capacity and symptoms in
patients with osteoarthrosis of the knee. Archives of Physical Medicine and Rehabilitation
83: 308–316.
Hamdan TA, Al-Essa KA (2003) Manipulation under anaesthesia for the treatment of frozen
shoulder. International Orthopaedics 27: 107–109.
Haug J, Wood LT (1988) Efficacy of neuromuscular stimulation of the quadriceps femoris
during continuous passive motion following total knee arthroplasty. Archives of Physical
Medicine and Rehabilitation 69: 423–424.
Healy WL, Seidman J, Pfeifer BA, Brown DG (1994) Cold compressive dressings after total
knee arthroplasty. Clinical Orthopaedics and Related Research 299: 143–146.
Heck D, Robinson R, Partridge C, Lubitz R, Freund D (1998) Patient outcomes after knee
replacement. Clinical Orthopaedics and Related Research 356: 93–110.
Herbert, R, Jamtvedt, G, Mead, J, Hagen, KB (2005) Practical Evidence-Based Physiotherapy
Edinburgh: Elsevier.
Hirsch HS, Lotke PA, MorrisonLD(1994) The posterior cruciate ligament in total knee surgery.
Save, sacrifice, or substitute? Clinical Orthopaedics and Related Research 309: 64–68.
Holt G, Dennis D (2003) The role of patella resurfacing in total knee arthroplasty. Clinical
Orthopaedics and Related Research 1: 76–83.
Insall JN, Ranawat CS, Scott WN, Walker P (1976) Total condylar knee replacement: preliminary
report. Clinical Orthopaedics and Related Research 120: 149–154.
Ivey M, Johnston RV, Uchida T (1994) Cryotherapy for postoperative pain relief following
knee arthroplasty. Journal of Arthroplasty 9: 285–290.
Jain NB, Guller U, Pietrobon R, Bond TK, Higgins LD (2005) Comorbidities increase complication
rates in patients having arthroplasty. Clinical Orthopaedics and Related Research
435: 232–238.
Jones DL,Westby MD, Greidanus N, Johanson NA, Krebs DE, Robbins L et al. (2005) Update
on hip and knee arthroplasty: current state of evidence. Arthritis & Rheumatism 53: 772–
780.
Kajino A, Yoshino S, Kameyama S, Kohda M, Nagashima S (1997) Comparison of results of bilateral total knee arthroplasty with and without patellar replacement for rheumatoid arthritis: a follow-up note. Journal of Bone and Joint Surgery 79A: 570.
Keblish PA, Varma AK, Greenwald AS (1994) Patellar resurfacing or retention in total knee arthroplasty: a prospective study of patients with bilateral replacements. Journal of Bone and Joint Surgery 76B: 930.
Kennedy DM, Stratford PW, Wessel J, Gollish JD, Penney D (2005) Assessing stability and change of four performance measures: a longitudinal study evaluating outcome following total hip and knee arthroplasty. BMC Musculoskeletal Disorders 6(3).
Kim BS, Reitman RD, Schai PA, Scott RD (1999) Selective patellar nonresurfacing in total knee arthroplasty: 10 year results. Clinical Orthopaedics and Related Research 367: 81.
Kramer JF, Speechley M, Bourne R, Rorabeck C, Vaz M. (2003) Comparison of clinic- and home-based rehabilitation programs after total knee arthroplasty. Clinical Orthopaedics and Related Research 410: 225–234.
Lachiewicz PF (2000) The role of continuous passive motion after total knee arthroplasty. Clinical Orthopaedics and Related Research 1: 144–150.
Lamb SE, Frost H (2003) Recovery of mobility after knee arthroplasty. Expected rates and influencing factors. Journal of Arthroplasty 18: 575–581.
Lingard EA, Bervan S, Katz JN, Kinemax Outcomes Group (2000) Management and care of patients undergoing total knee arthroplasty: variations across different health care settings. Arthritis Care and Research 13: 129–136.
Lorentzen JS, Petersen MM, Brot C, Madsen OR (1999) Early changes in muscle strength after total knee arthroplasty. Acta Orthopedica Scandinavica 70: 176–179.
March LM, Cross M, Tribe KL, Lapsley HM, Courtenay BG, Cross MJ et al. (2004) Two knees or not two knees? Patient costs and outcomes following bilateral and unilateral total knee joint replacement surgery for OA. Osteoarthritis Cartilage 12: 400–408.
MarchLM,Cross MJ, LapsleyH, Brnabic AJ,Tribe KL, BachmeierCJMet al. (1999) Outcomes
after hip or knee replacement surgery for osteoarthritis. Medical Journal of Australia 171:
235–238.
Martin SS, Spindler KP, Tarter JW, Detwiler KB (2002) Does cryotherapy affect intraarticular
temperature after knee arthroscopy? Clinical Orthopaedics and Related Research 1: 184–
189.
McAuley JP, HarrerMF, Ammeen D, EnghGA(2002) Outcome of knee arthroplasty in patients
with poor pre-operative range of motion. Clinical Orthopedics and Related Research 404:
203–207.
McDonald S, Hetrick S, Green S (2004) Pre-operative education for hip or knee replacement.
Cochrane Library 1 http://www.thecochranelibrary.com CD003526.
Milne S, Brosseau L, Robinson V, Noel MJ, Davis J, Drouin H et al. (2003) Continuous
passive motion following total knee arthroplasty. Cochrane Library 2 http://
www.thecochranelibrary.com CD004260.
Mitchell C, Walker J, Walters S, Morgan AB, Binns T, Mathers N (2005) Costs and effectiveness
or pre- and post-operative physiotherapy for total knee replacement: randomized
controlled trial. Journal of Evaluation in Clinical Practice 11: 283–292.
Mizner RL, Stevens JE, Snyder-Mackler L (2003)Voluntary activation and decreased force production
of the quadriceps femoris muscle after total knee arthroplasty. Physical Therapy
83: 359–365.
Mizner RL, Petterson SC, Stevens JE, Axe MJ, Snyder-Mackler L (2005) Preoperative quadriceps
strength predicts functional ability one year after total knee arthroplasty. Journal of
Rheumatology 32: 1533–1539.
Moffet H, Collet J-P, Shapiro SH, Paradis G, Marquis F, Roy L (2004) Effectiveness of intensive
rehabilitation on functional ability and quality of life after first total knee arthroplasty: a
single-blind randomised controlled trial. Archives of Physical Medicine and Rehabilitation
85: 546–556.
Munin MC, Rudy TE, GlynnNW, Crossett LS, Rubash HE (1998) Early inpatient rehabilitation
after elective hip or knee arthroplasty. Journal of the American Medical Association 279:
847–852.
Australian Bureau of Statistics (1995) National Health Survey SF-36 Population Norms Canberra:
Australian Bureau of Statistics, Cat. No. 4399.0.
Naylor JM, Ireland JE, Mohammed M (2005) Outcomes Following Primary THR and TKR:
part 1 – acute and short-term outcomes Sydney: Whitlam Joint Replacement Centre,
SSWAHS.
Naylor JM, Fransen M, Ireland JE, Winstanley J (2006a) Outcomes Following Primary THR
and TKR: part 2 – longer-term outcomes. Sydney: Whitlam Joint Replacement Centre,
SSWAHS.
Naylor JM, Harmer AR, Fransen M, Crosbie J, Innes L (2006b) The status of physiotherapy
rehabilitation following total knee replacement in Australia. Physiotherapy Research
International 11: 35–47.
NHMRC and ASBT (2001) Clinical practice guideline on the use of blood components.
http://www.nhmrc.health.gov.au/.
Oldemeadow L, McBurney H, Robertson V (2001) Hospital stay and discharge outcomes after
knee arthroplasty. Journal of Quality and Clinical Practice 21: 56–60.
Ouellet D, Moffet H (2002) Locomotor deficits before and two months after knee arthroplasty.
Arthritis & Rheumatism 47: 484–493.
Paul RG, Bailey AJ (1996) Glycation of collagen: the basis of its central role in the late
complications of ageing and diabetes. International Journal of Biochemistry and Cell
Biology 28: 1297–1310.
Pearson S, Moraw I, Maddern GJ (2000) Clinical pathway management of total knee arthroplasty:
a retrospective comparative study. ANZ Journal of Surgery 70: 351–354.
Petterson SC, Mizner RL, Snyder-Mackler L (2003) Factors that influence six-minute walk in
individuals after total knee arthroplasty. Journal of Geriatric Physical Therapy 26: 50.
Pierson J, Earles D,Wood K (2003) Brake response time after total knee arthroplasty. Journal
of Arthroplasty 18: 840–843.
Rajan RA, PackY, Jackson H, Gillies C, AsirvathamR(2004)Noneed for outpatient physiotherapy
following total knee arthroplasty: a randomized trial of 120 patients. Acta Orthopedica
Scandinavica 75(1): 71–73.
Rand JA (1996) Posterior cruciate retaining total knee arthroplasty. In: Morrey BF (ed.) Reconstructive
Surgery of the Joints Volume 2 (2 edn) New York: Churchill Livingstone, pp.
1401–1408.
Ritter MA, Berend ME, Meding JB, Keating EM, Faris PM, Crites BM (2001) Long-term follow-up of anatomic graduated components posterior cruciate-retaining total knee replacement. Clinical Orthopedics 388: 51–57.
Rodriguez JA, Bhende H, Ranawat CS (2001) Total condylar knee replacement: a 20-year follow-up study. Clinical Orthopaedics and Related Research 388: 10–17.
Roos E (2003) Effectiveness and practice variation of rehabilitation after joint replacement. Current Opinion in Rheumatology 15: 160–162.
Rossi MD, Hasson S (2004) Lower-limb force production in individuals after unilateral total knee arthroplasty. Archives of Physical Medicine and Rehabilitation 85: 1279–1284.
Salmon P, Hall G, Peerbhoy D, Shenkin A, Parker C (2001) Recovery from hip and knee arthroplasty: patients’ perspective on pain, function, quality of life, and well-being up to 6 months postoperatively. Archives of Physical Medicine and Rehabilitation 82: 360–366.
Scarcella JB, Cohn BT (1995) The effect of cold therapy on the postoperative course of total hip and knee arthroplasty patients. American Journal of Orthopedics November: 847– 852.
Segal L, Day SE, Chapman AB, Osborne RH (2004) Can we reduce disease burden from osteoarthritis? An evidence-based priority-setting model. Medical Journal of Australia 180: 11S–17S.
Shields RK, Enloe LJ, Leo KC (1999) Health related quality of life in patients with total hip or knee replacement. Archives of Physical Medicine and Rehabilitation 80: 572–579.
Shumway-Cook A, Brauer S, Woollacott M. (2000) Predicting the probability for falls in community-dwelling older adults using the timed up and go test. Physical Therapy 80: 897–903.
Sigal RJ, Kenny GP, Wasserman DH, Castaneda-Sceppa C (2004) Physical activity/exercise and type 2 diabetes. Diabetes Care 27: 2518–2539.
Skinner J, Weinstein JN, Sporer SM, Wennberg JE (2003) Racial, ethnic, and geographic disparities in rates of knee arthroplasty among Medicare patients. New England Journal of Medicine 349: 1350–1359.
Smith J, Stevens J, Taylor M, Tibbey J (2002) A randomised controlled trial comparing compression
bandaging and cold therapy in postoperative total knee replacement surgery. Orthopaedic Nursing 21: 61–66.
Steffen T, Hacker TA, Mollinger L (2002) Age- and gender-related test performance in community-dwelling elderly people: six-minute walk test, berg balance scale, timed up & go test, and gait speeds. Physical Therapy 82: 128–137.
Stratford PW, Kennedy D, Pagura SM, Gollish JD (2003) The relationship between self-report and performance-related measures: questioning the content validity of timed tests. Arthritis & Rheumatism 49: 535–540.
Straw R, Kulkarni S, Attfield S, Wilton TJ (2003) Posterior cruciate ligament at total knee
replacement. Essential, beneficial or hindrance? Journal of Bone and Joint Surgery 85B: 671–674.
van Essen GJ, Chipcase LS, O’Connor D, Krishnan J (1998) Primary total knee replacement: short-term outcomes in an Australian population. Journal of Quality in Clinical Practice 18: 135–142.
Walsh M, Woodhouse LJ, Thomas SG, Finch E (1998) Physical impairments and functional limitations: a comparison of individuals 1 year after total knee arthroplasty with control subjects. Physical Therapy 78: 248–258.
Wang A, Hall S, Gilbey H, Ackland T (1997) Patient variability and the design of clinical pathways after total hip replacement surgery. Journal of Quality in Clinical Practice 17: 123–129.
Webb JM, Williams D, Ivory JP, Day S, Williamson DM (1998) The use of cold compression dressings after total knee replacement: a randomised controlled trial. Orthopedics 21: 59–61.
Whiteside LA (1995) Ligament balancing and bone grafting in total knee replacement of the varus knee. Orthopedics 18: 117–122.
Whiteside LA (1999) Selective ligament release in total knee arthroplasty of the knee in valgus. Clinical Orthopedics and Related Research 367: 130–140.
Whiteside LA. (2005) Assess and release the tight ligament. In: Bellemans J, Ries MD, Victor J (eds) Total Knee Arthroplasty: a guide to get better performance, pp. 170–176.
Wood DJ, Smith AJ, Collopy D, White B, Brankov B, Bulsara MK (2002) Patellar resurfacing in total knee arthroplasty: a prospective, randomized trial. Journal of Bone and Joint Surgery 84A: 187–193.
JUSTINE NAYLOR, ALISON HARMER AND RICHARD WALKER
CASE REPORT
Mrs JM, a 70 year old female, presented pre-operatively with severe tri-compartmental osteoarthritis (OA) of her right knee. On examination, she was obese (Body Mass Index (BMI) 30.8), walked with a varus thrust and a marked limp on the right, and used a walking stick. Her gait, lower limb strength, and range of motion (ROM) profiles were as follows:
Gait speed:
– Timed up-and-go (TUG) – 15 seconds.
– Timed 15-m walk – 21 seconds (0.71 m/s).
– 6-min. Walk Test (6 MWT), 322m, limited by knee pain (right > left).
Isometric strength at 90◦:
– Knee extensors: Right, 106 Newtons; Left, 150 Newtons.
– Knee flexors: Right, 58 Newtons; Left, 100 Newtons.
Knee range of motion (ROM) (passive, supine):
– Right=−10◦ to 100◦; Left=−5 ◦ to 105◦.
Symptomatically, Mrs JM reported high pain (13/20), stiffness (5.8/5), and difficulty (45.5/68) scores on the WOMAC1 subscales, and poor bodily pain (30/100) and physical function (26.6/100) scores on the SF-362 domains.
In terms of Mrs JM’s medical history, she reported bilateral knee OA (right > left) of idiopathic origin of eight year’s duration. She suffered from hypertension (which was controlled), ischaemic heart disease (IHD), and demonstrated poorly controlled type 2 diabetes mellitus (HbA1c (glycosylated haemoglobin) 8.2 %) of seven years’ duration. Consequently, her American Society of Anesthesiologists (ASA) anaesthetic risk score was estimated as II. Consequent to her multiple co-morbidity status, her medication usewas extensive; for her pain management in particular, a poly-pharmacy approach was evident:
1 Western Ontario & MacMaster Universities Osteoarthritis Index (low scores indicating better status).
2 Medical Outcome Study, Short Form-36 Health related quality of life scale (high scores indicating better
Carvedilol, 25 mg daily.
Glyceryl trinitrate, patch 25 mg daily.
Metformin, 1 g bd.
Paracetamol, prn.
Celecoxib, 200 mg daily.
Glucosamine and chondroitin sulphate.
Her haemoglobin concentration (Hb) was noted to be 139 g/l.
As part of routine anaesthetic work-up.
Socially, Mrs JM lived with her spouse in a house with 18 stairs. She had ceased recreational lawn bowls six months prior to her presentation due to pain and giving way in her right leg. She was a pensioner, reporting a low income level throughout her family life, and the highest level of education attainedwas primary (elementary) level.
INTRODUCTION
The benefits of total knee arthroplasty (TKA) for the individual with arthritis are perceived relatively quickly (usually within three to six months) and are generally pluralistic, including improvements in pain, ROM, knee stability, mobility, function, and health-related quality of life (HRQoL) (Aarons et al. 1996 A; Ethgen et al.
2004 A; Fortin et al. 2002 A; March et al. 1999 A; March et al. 2004 A; McAuley et al. 2002 A; Naylor et al. 2006a A; Pierson et al. 2003 A; Salmon et al. 2001 A; Van Essen et al. 1998 A). Consequently, TKA is estimated to be a highly cost-effective treatment option for severe arthritis (Segal et al. 2004 A). Largely ignored in costbenefit calculations, however, are the costs associated with ongoing (post-acute care)
rehabilitation. Such costs can indirectly be appreciated via the findings of March et al. (2004 A), who reported that the average number of out-patient physiotherapy visits by primary TKA patients was 10 in the first post-operative year, exceeding the average number of patient visits to any other health professional. This, of course, was in addition to any acute in-patient rehabilitation provided during the in-patient period
(an average of 12 days) and, for many (33 %), treatment in a rehabilitation facility.
We anticipate that the findings by March et al. are readily generalised as we have observed that referral to ongoing physiotherapy post-TKA is fairly routine in Australia, with out-patient based treatment predominating (Naylor et al. 2006bA). Our findings, obtained through a nationwide survey of TKA rehabilitation providers, echo earlier observations by Lingard et al. (2000A), who reported the frequent utilisation of ongoing physiotherapy post-TKA in the UK, Australia and the US, with the latter tending to rely more on in-patient services. Given that the numbers of TKA procedures have doubled in these same countries over the last decade (Australian Orthopaedic Association National Joint Replacement Registry 2004 A; Dixon et al. 2004 A; Skinner et al. 2003 A), the volumes of patients potentially requiring ongoing rehabilitation to supplement surgery must also have increased. Anecdotally, in Australia at least, there is a perception that the increased surgical throughput has not been accompanied by increases or appropriate increases in the availability of downstream (ward-based and rehabilitative) resources. This must translate at some point into a time-squeeze at the therapist-patient interface and access-block for rehabilitation services. For these reasons, the need to understand the costs and benefits of rehabilitation should be an urgent priority for health systems worldwide. Osteoarthritis (OA), the leading precipitant for TKA, is associated with significant loss of lower limb muscle strength (Fransen et al. 2003 A; Gur et al. 2002 A), walking speed (Gur et al. 2002 A; Lamb & Frost 2003 A), and function (Fransen et al. 2001 A). Exercise programmes involving patients with OA have repeatedly been shown to elicit significant yet small improvements in these parameters within relatively short time frames (for example, at two months) (see reviews by Bischoff & Roos 2003 R; Fransen et al. 2001 R). In contrast, TKA – a procedure typically reserved for recalcitrant arthritis – does not guarantee immediate improvements in these same parameters. Though significant improvements do occur early, several cross-sectional (Berth et al. 2002 A; Mizner et al. 2003 A; Walsh et al. 1998 A) and longitudinal (Benedetti et al. 2003 A; Lamb & Frost 2003 A; Lorentzen et al. 1999 A; Ouellet & Moffet 2002 A; Salmon et al. 2001 A) studies reveal shortfalls in gait, strength, and quality of life, compared to age-matched controls, several months to years after surgery. The argument for ongoing rehabilitation following TKA, therefore, is based on the following related contentions:
That age-predicted norms for muscle function, gait patterns, and physical activity levels are not spontaneously or completely achieved post-surgery, and;
That short-term exposure to prescribed interventions or physical activities will facilitate more complete recovery.
Given that the provision of acute and ongoing physiotherapeutic rehabilitation appears to be standard care across several countries, it is staggering to realise that the evidencebase which underpins rehabilitation in this area is tenuous. While there are considerable bodies of work supporting some, but not all, physiotherapeutic interventions in the acute ward phase, there is comparatively little evidence to support the various modes of ongoing rehabilitation offered either in the community or in rehabilitation wards. The trials that have been conducted (Frost et al. 2002 A; Kramer et al. 2003 A; Moffet et al. 2004; Rajan et al. 2004 A) all compared one mode of ongoing physiotherapy to another and did not include a true non-interventional control. Thus, the contribution of rehabilitation per se to the overall recovery process is uncertain.The lack of definitive evidence is problematic for policy makers worldwide, as health service providers are increasingly required to justify the high costs of health care, while the demand for services (in this case, rehabilitation) is increasing through sheer volume alone. Furthermore, the lack of evidence is problematic at the coalface, given that variation in practice is likely to be (Roos 2003C), and has been observed to be (Naylor et al. 2006b A), the rule, further undermining our capacity to identify best practice.
This chapter addresses questions concerning the efficacy of various acute physiotherapeutic interventions and longer-term rehabilitative strategies Mrs JM may be exposed to through her journey of recovery. Questions concerning the impact of prosthesis type or specific surgical choices on the potential to rehabilitate or the mode of rehabilitation required are also briefly addressed. Mrs JM presents fairly typically for an elderly patient awaiting TKA for severe knee OA (Ackerman et al. 2005 A; Bozic et al. 2005 A; Heck et al. 1998 A; March et al. 2004 A; Mizner et al. 2003 A; Naylor et al. 2006a A; Ouellet & Moffet 2002 A). Notably, the measured variables are frequently utilised and recommended for the evaluation of OA and TKA (Bellamy et al. 1988 A; Ethgen et al. 2004 R; Fransen et al. 2003 A; Kennedy et al. 2005 A; March et al. 1999 A; March et al. 2004 A; Ouellet & Moffet 2002 A; Petterson et al. 2003 A). Compared to norm data or age-matched controls (see Table 1),
Table 1. Normative or age-matched physical and health-related quality of life data
Australian Age-Matched
Norm Data Control Data
Physical Function
SF-36 Physical Function 65.2 1 —
WOMAC Physical Function NA —
Walking Speeds
Timed up-and-go (sec) — 8–11 2,3,4
15-m walk (m/sec) — 1.33–1.84 2,5
6-minute walk (m) — 448 2
Isometric Muscle Strength
Knee Extensors (N) — 225 (sd 49)6
Knee Flexors (N) — 139 (36)6
Health-Related Quality of life
SF-36 General Health 64.1 —
SF-36 Vitality 60 —
SF-36 Mental Health 75.3 —
Knee Range of Motion
Total — 143◦4
Pain Scores
SF-36 Bodily Pain 69 —
WOMAC Pain NA —
Legend: 1National Health Survey SF-36 Population Norms, ABS 1995 (unstandardised mean scores, female); 2Steffen et al. 2002 A; 3Ouellet & Moffet 2002 A; 4Shumway-Cook et al. 2000 A; 5Walsh et al. 1998 A; 6Fransen et al. 2003A; NA=not available at time of publication (Australian data). Normative data from large population sets are provided where available; otherwise, age-matched data, sourced from relevant osteoarthritis or knee replacement trials, are cited.
The reported daily consumption of analgesic and anti-inflammatory medications is consistent with the high pain scores, and the use of a walking aid is somewhat typical for degenerative joint disease. It is important to note that our own experiences indicate the analgesic, anti-inflammatory, and walking aid profiles are not, in isolation, reliableindicators of severity or improvement, as behavioural factors greatly influence their use.
The patient’s co-morbidity profile is also typical for this patient population, with hypertension in particular being the most common co-morbidity observed in several TKA cohorts (Denis et al. 2006 A; Moffet et al. 2004 A; Naylor et al. 2005 A). Additionally, some physiological limitation is qualitatively suggested by the ASA score, again not atypical of TKA recipients (Bozic et al. 2005A; Naylor et al. 2005aA; Pearson et al. 2000 A). Given the self-exertion nature of many rehabilitation interventions, recognising the physiological limitations imposed by concurrent illnesses is an essential consideration in any rehabilitation programme. Likewise, the socioeconomic factors, highlighted as low income and education levels, are associated with poorer pre-operative function (Ackerman et al. 2005 A) and some post-surgical outcomes (Fortin et al. 1999 A). For the therapist, these factors become relevant when setting realistic long-term patient goals and when benchmarking rehabilitation outcomes between surgical units.
OPERATIVE HISTORY AND ACUTE POST-OPERATIVE
PRESENTATION
Relevant operative details:
General anaesthetic + femoral and sciatic nerve blocks.
Tri-compartmental primary TKA.
Cemented femoral, tibial, and patella components.
Fixed-bearing, increased congruency, polyethylene bearing.
Posterior cruciate ligament (PCL) sacrificed.
Release of medial collateral ligament.
Anterior cruciate ligament (ACL) removed.
Intra-articular low suction wound drain in situ.
Presentation 18 hrs post-op (Day 1):
Symptoms:
– Reporting 2/10 pain on visual analogue scale, using patient-controlled analgesia c/o numbness and lack of movement in foot.
Mobility:
– In bed, awaiting assessment by physiotherapist.
ROM:
– Start flexion, –10◦.
– End flexion, 60◦.
– Restricted by oedema and crepe bandaging.
– Quadriceps lag, 15◦.
Vital observations:
– BP 110/70 (normally 130/80).
– HR 95–100.
– RR 18.
– SpO2 97 % (3 L/min. O2, nasal prongs).
Blood results:
– Hb 105 g/l.
– Blood glucose level (BGL) 7.7 mmol·l−1.
Other medication:
– Anti-hypertensives and metformin withheld.
– Twice daily protaphane, with top up sliding scale to maintain blood glucose control.3
GENERAL PRINCIPLES
Rehabilitation in the acute phase is largely directed towards the minimisation of the effects of surgical trauma and rendering the patient safe for discharge. The rehabilitative strategies include the use of modalities and techniques to reduce intra- and extra-articular oedema, improve or maintain knee ROM, offset the adverse effects of bed rest, and assist independent ambulation. With respect to the determination of discharge readiness, it is recognised that some surgical units specify a minimum flexion ROM before a patient is deemed fit (Ganz & Benick 2004 Abstract), while others rely more on the level of function achieved (Munin et al. 1998 A; Naylor et al. 2006b A). Though speculative, the latter approach may have evolved secondary to an ever-present need to maintain patient flow in order to keep wait lists in check. In this context, the need to achieve specific physical milestones, such as a minimum flexion requirement, becomes less urgent (Benick et al. 2004 Abstract). It is also recognised that the threshold for discharging patients to an in-patient rehabilitation unit may differ between surgical units, with a lower threshold likely in the private market.
The nature and timing of acute care rehabilitation has also been altered over the last 10 years via the introduction of specific multi-disciplinary care pathways (protocols). Such pathways have procured impressive (up to 50 %) decreases in acute length of stay (LOS) (Brunenberg et al. 2005 A; Dowsey et al. 1998 A; Munin et al. 1998 A; Pearson et al. 2000 A;Wang et al. 1997 A), which must inevitably impact on the goals of rehabilitation, as the therapist-patient interface has contracted considerably at ward level. Finally, central to effective rehabilitation both now and in the longer-term, is good pain management. It is beyond the scope of this chapter to review the evolution of pain management in this context, however; suffice it to say that physiotherapists act as barometers of good pain control in their estimation of whether a patient can engage in their rehabilitation effectively.
3 Additionally, referral to an endocrinologist was initiated on admission, and the recommendation was to add 1/2 80 mg tab of gliclazide twice daily once metformin is recommenced, with the option to increase to 80 mg twice daily if needed (i.e. if HbA1c remains high).
The sources of evidence reviewed for specific rehabilitative interventions in theacute phase consisted of RCTs and systematic reviews. In order to identify the relevant literature, the following combinations of terms were used in an electronic literature
search of MEDLINE, CINAHL and EMBASE:
Arthroplasty, knee, Cryotherapy.
Arthroplasty, knee, CPM.
Arthroplasty, knee, walking aid progression.
Arthroplasty, knee, exercises.
Studies were considered appropriate if the subjects had undergone primary TKA, were randomised to receive the treatment(s) under investigation, and the treatment(s) was (were) conducted in the acute in-hospital phase. In cases where a systematic review existed for a given intervention, this predominantly formed the basis for the review, to avoid duplication. Studies focusing on multi-disciplinary and multi-faceted clinical pathways were generally not included. Only studies written in English were reviewed. This review does not include the effects of pre-operative programmes on outcomes. For these, the following reviews are recommended: Ackerman et al. 2004 R; McDonald et al. 2004 R.
QUESTION 1
Does cryotherapy work?
External cooling of the knee surfaces has been shown, in the absence of haemarthrosis, to lower intra-articular temperatures in humans by 2.7–5 ◦C (Martin et al. 2002A).
This, together with the local effects of cold therapy on neural and vascular function, presumably motivates the use of cryotherapy post-TKA for the purposes of reducing pain and swelling. The use of cryotherapy has been observed to be inconsistent in the acute phase following TKA, in terms of both the factors governing its application (Barry et al. 2003) and whether it is utilised at all (Naylor et al. 2005 A, 2006b A).
To date, cryotherapy post-TKA has not been systematically reviewed, but several RCTs have been conducted (Gibbons et al. 2001 A; Healy et al. 1994 A; Ivey et al. 1994 A; Scarcella & Cohn 1995 A; Smith et al. 2002 A; Webb et al. 1998 A). Only one study (Webb et al. 1998 A), comparing cold compression to a non-interventional control, observed significantly less blood transfusions, analgesic consumption, and pain with cold therapy. Of course, the contribution made by the compression component could not be differentiated in this study. Of note, despite the pain relief and blood loss benefits, no differences in ROM acutely or at 12 weeks were observed.
For the majority of the remaining studies in this area, no or minor differences have been observed between those receiving and not receiving early cryotherapy on several outcomes, including LOS, transfusion needs, swelling, ROM, pain, and analgesic use.
Having said this, the interpretation of the impact of cryotherapy in these studies is clouded by comparisons with alternative treatments (such as compression bandaging or alternative cold therapy) (Gibbons et al. 2001 A; Healy et al. 1994 A; Smith et al.2002 A) rather than comparisons with true non-interventional controls Healy and colleagues (1994 A) compared cryotherapy to ice packs. Smith et al. (2002 A) used cold therapy in both groups after 24 hours. Scarcella and Cohn (1995 A), with their sample of 24 TKA patients, were not likely to have had sufficient power to detect differences between their groups when others (Smith et al. 2002 A) have required a sample of 80 for the same outcome variables. Finally, Gibbons et al. (2001 A) did not account for possible gender differences in Hb levels between the treatment and control groups, which themselves differed in their gender profile. This may have explained why cold compression was not associated with a lower transfusion requirement in this study despite being associated with smaller post-operative blood losses. Even with the lack of irrefutable evidence demonstrating that there is no additional benefit from cryotherapy, various authors (Healy et al. 1994 A; Smith et al. 2002 A) have concluded that its costs outweigh its benefits and that compression is preferred in light of this.We conclude that although at this stage it would appear that cryotherapy offers no additional benefits beyond those which could be achieved with compression alone, the methodological limitations of the majority of studies conducted render this issue unresolved.
Regarding Mrs JM, the available evidence does not strongly support or refute the use of cryotherapy, nor is it clear whether compression bandaging alone is superior to it. Thus, the therapist would be justified in trying either. Ideally these modalities would be applied both before and after physiotherapy; at the very least, pain, oedema and ROM should be monitored pre- and post-application. However, Mrs JM’s initial numbness – presumed at this stage to be a hangover from her intra-operative regional anaesthetic – may delay the commencement of ice therapy. Of course, neural deficits beyond 24 hours will need to be differentiated from possible chronic loss due to diabetic neuropathy. Though speculative at this point, the presence of the haemarthrosis following TKA may undermine the impact of external ice applications, rendering the effects of compression bandaging more important.
QUESTION 2
Does continuous passive motion work?
Continuous passive motion (CPM), like cryotherapy, is an adjunctive rehabilitation tool intended to decrease swelling and haemarthrosis, and enhance soft tissue healing and joint ROM (Milne et al. 2003 A). In contrast to cryotherapy, however, CPM has been subject to many RCTS involving TKA recipients (n=59), one Cochrane review (Milne et al. 2003 A), and one qualitative review (Lachiewicz 2000 R). Thus, more definitive conclusions can be drawn regarding its effectiveness.
Milne et al. (2003 A), based on a meta-analysis, concluded that CPM combined with standard physiotherapy was associated with a small increase in flexion ROM at two weeks (4.3◦ weighted mean difference (WMD4)), decreased LOS (0.69 days WMD),and a decreased risk of manipulation within the first month (relative risk 0.12).
4 WMD: difference between control and treatment group is weighted by the inverse of the variantion.
CPM was not found to improve passive ROM. The authors did conclude, however, that information and protocol biases were present in the review due to inadequate reporting of some variables (for example, whether ROM was passive or active) and inconsistent protocols (for example, pain relief and pre-operative education) across studies. Information on ideal dose and application could not be derived. In light of these facts, the authors recommended that the potential benefits of CPM be weighed against the possible increased costs and inconvenience, and that more research be conducted to determine the optimum treatment parameters. Not included in the analyses were the effects of CPM on midline wound healing, bleeding overall, and hospital costs. These have been shown to be a concern in some trials (Lachiewicz 2000 R).
Since the publication of the meta-analysis by Milne and colleagues, only one other RCT has been conducted in TKA patients. Denis et al. (2006 A) did not observe any differences in discharge (∼ eight days post) ROM, LOS,WOMAC function, and TUG times between those treated with conventional physiotherapy plus 35 or 120 minutes of CPM daily, and those receiving conventional physiotherapy only.With the exception of LOS, these results confirm the conclusions of the aforementioned metaanalysis.
It is unfortunate, however, that the number of manipulations post-discharge was not monitored given that this is perhaps the most clinically relevant outcome concerning CPM. In terms of current clinical practice, we observed that CPM does not appear to be in routine use in Australia (Naylor et al. 2006b A). Whether this is the case elsewhere is unknown as there are no other survey data concerning this. We also observed in our unit, where CPM was routinely prescribed, that only 40 % of patients received it (Naylor et al. 2005 A). Protocol deviance was explained by a combination of lack of awareness of the protocol by rotating physiotherapists, and their perceived lack of need – the latter possibly explained by the fact that functionality and not ROM primarily determines discharge at our unit. At this point in time, our CPM practices, together with our pain relief and pre-operative education policies, are under review, as the number of manipulations performed within six months of surgery has increased in recent times.
Regarding Mrs JM, in view of the risk of manipulation alone, CPM should be initiated at least once per day for several hours during bed rest periods. This recommendation ideally applies to units where CPM is readily available and where medical and nursing staff can apply it. Though speculative, CPM may be of particular benefit to Mrs JM given her poorly controlled diabetes (evidenced by the elevated HbA1c of 8.2 %; non-diabetic range 3–6 %). Glycosylation (permanent protein modification by glucose) of collagen or elastin as a result of persistently high BGL may result in tissue stiffness (Paul & Bailey 1996 B), hence Mrs JM may be at a greater risk of manipulation.5
5 22%of patients presenting for manipulation under anaesthesia for frozen shoulders had diabetes (Hamdan
& Al-Essa 2003).
QUESTION 3
What is the evidence for exercise and early ambulation to improve ROM, decrease length of stay, and prevent deep venous thrombosis?
Only one study has compared the outcomes of patients who received formal knee flexion exercises in addition to standardised physiotherapy with those who received standardised physiotherapy only (Ganz & Ranawat 2004 Abstract). Though formal knee flexion exercises were associated with greater active knee flexion at one week, this did not translate into any functional differences (such as stair ambulation or use of aids) or shorter LOS. At three and 12 months, there were no differences in active knee flexion. No studies were found focusing on active knee extension. Despite the lack of evidence in support of specific active exercises, we have observed the prescription of lower limb exercises in the acute stage to be routine in Australia (Naylor et al. 2006b A). This notwithstanding, as there does not appear to be any routine case to suggest active exercises are detrimental in this patient group, we find no reason for not including them in the therapy repertoire.
Similarly to active exercises, the arguments for early ambulation post-TKA rest largely on the desire to minimise the well-known adverse effects of bed rest and to accelerate discharge from hospital. To our knowledge, only one RCT has been conducted (Munin et al. 1998 A) which highlights the specific benefits of early rehabilitation, including early ambulation (commencing Day Three versus Day Seven), on LOS, functional performance, and Deep Vein Thrombosis rate. Though the specific contribution attributable to early ambulation alone cannot be reliably estimated, the absence of evidence to the contrary suggests protocols aimed at early ambulation are desirable. We do qualify this statement, however, in that we recommend an assessment of the patient’s medical stability (including blood pressure, heart rate and rhythm, BGL, oxygen saturation levels, and Hb) precedes any physiotherapy intervention.
Regarding Mrs JM, her lower limb neural deficit will preclude ambulation and some bed exercises until it resolves. A combination of closed- and open-chain isometric, concentric, and eccentric exercises will be prescribed for the flexor and extensor muscle groups in her lower limbs. Ambulation will commence after removal of the wound drains. Her cardiovascular history necessitates close monitoring of her vital signs prior to her participating in any exercise, however. Her low Hb is typical at this stage, given the acute blood losses (mean 608 mls) associated with the surgery (Naylor et al. 2005 A), and, at her current level, does not warrant a transfusion (NH&MRC & ASBT 2001 A).
QUESTION 4
What evidence guides walking aid progression?
The literature search yielded no RCTs investigating the optimal rate of walking aid progression. We are aware of surgical units that dictate the rate of progression according to the presence or absence of cement. In our unit, all patients are progressed and discharged on crutches, with instructions to weight-bear as tolerated unless otherwise indicated. It is not clear at this stage whether the rate of progression onto a walking stick or to complete independence from walking aids is a concern for longterm prosthesis stability, the restoration of normal gait patterns, or the evolution of back pain.
QUESTION 5
Does electrical stimulation work?
The electrical stimulation of the knee extensor muscles post-TKA is based on the premise that voluntary activation is not sufficient to restore strength (Avramidis et al. 2003A). Three studies were identified that randomised the use of electrical stimulation to the vastus medialis or quadriceps femoris during CPM, commencing in the acute period and given alongside a standardised physiotherapy programme. Gotlin et al. (1994 A) and Haug and Wood (1988 A) observed that patients receiving two to three hours of muscle stimulation daily until discharge experienced less extensor lag and shorter LOS. In a longer-term study, Avramidis et al. (2003 A) observed that patients receiving electrical stimulation for two hours twice daily from the second post-operative day for six weeks, attained a faster walking speed at six weeks, and this effect carried over until the 12th week. The authors concluded that the greater walk speed was a consequence of more rapid quadriceps recovery and, as such, a greater ability to participate in exercise. It should be noted that the control group did not receive any standardised physiotherapy post-discharge. The addition of a third group that received standardised physiotherapy for six weeks would have helped to clarify whether electrical stimulation was superior to or simply a replacement for voluntary muscle activation. While the use of electrical stimulation looks promising, the technical and potentially cumbersome nature of the procedure, and the prerequisite for effective communication between patient and therapist for safety reasons, may have deterred widespread adoption of this treatment option.
Regarding Mrs JM, assuming availability of the device and competency of both the staff and patient in its use, intermittent neuromuscular stimulation is an appropriate rehabilitation intervention, given her quadriceps lag.
QUESTION 6
What is the evidence for hydrotherapy?
No RCTs were identified concerning the efficacy of hydrotherapy post-TKA. We recognise that it is a treatment option where facilities exist (Naylor et al. 2006b A) and that a non-randomised trial has been conducted in Germany (Erler et al. 2001 A).
No recommendations can be made at this stage, but note that, at the very least, the integrity of thewound is paramount for hydrotherapy to be considered a viable option. REHABILITATION IN THE POST-DISCHARGE PHASE
GENERAL PRINCIPLES
The post-discharge phase of rehabilitation commences after discharge from an acute care facility. Goals of rehabilitation in the earlier post-discharge phase focus upon increasing the level of independence of the patient, which may include weaning off a walking aid, maintaining and improving knee joint ROM, controlling or reducing residual oedema, increasing muscle strength and endurance, and gradual return to work and leisure activities. In the later phase of post-discharge rehabilitation, goals include further improvement of muscle strength and endurance, improvement of cardiovascular fitness, and full return to work and leisure activities.
The sources of evidence reviewed for specific rehabilitative interventions in the post-acute phase consisted of RCTs and systematic reviews. In order to identify the relevant literature, the following combinations of terms were used in an electronic literature search of MEDLINE, CINAHL and EMBASE:
Total knee replacement, with subject headings: arthroplasty, replacement, knee; knee prosthesis; TKR. Rehabilitation, with all subject headings. Physiotherapy, with subject headings: exercise therapy; orthopedics; physical therapy (specialty); physiotherapy. The initial literature search yielded 230 studies. For the present review, studies were only included if the subjects had undergone primary TKA, were randomised to receive the treatment(s) under investigation, and the treatment(s) was (were) conducted in the post-acute phase. Only studies written in English were reviewed. Only five trials satisfied these criteria, thus revealing the paucity of evidence for effects of rehabilitation in the post-acute phase. One study (Mitchell et al. 2005 A) included pre-operative physiotherapy in one group and was thus excluded. The remaining four trials differed markedly in their methodology and investigated the effects of out-patient physiotherapy versus home-based rehabilitation (Kramer et al. 2003 A; Rajan et al. 2004 A); traditional versus functional home-based exercise (Frost et al. 2002 A); and intensive versus usual care treatment (Moffet et al. 2004 A). Due to the limited number of studies identified and the holistic nature of the physiotherapy programmes described, it was not possible to examine the effect of a single treatment component in the postacute phase. In addition to the five reports of randomised trials, one recent review that presented current evidence from experts on knee and hip arthroplasty was identified (Jones et al. 2005 R).
QUESTION 7
What is the evidence supporting early post-discharge rehabilitation?
Three RCTs have examined the effects of physiotherapy provided in the early postdischarge phase of rehabilitation; that is, commencing immediately after discharge from acute care. Kramer et al. (2003 A) investigated effects of clinic- versus homebased rehabilitation. All patients were provided with advice on knee management and were prescribed home strengthening and ROMexercises, the basic form of which they were taught during the acute in-patient period. The home-based group received weekly phone calls from a physiotherapist, whereas patients in the clinic-based group attended the clinic once or twice weekly until three months post-operation. At three and 12 months post-operation there was no difference between groups on any outcome measures, which included WOMAC total and pain and function subscales, SF-36 total, knee flexion range, 30-second stair test, and 6MWT. Similarly, another study (Rajan et al. 2004 A) found no additional benefit of out-patient physiotherapy compared with a home exercise programme at three, six, or 12 months; however, there was no description of the physiotherapy interventions, and the only outcome measure reported was knee flexion range. Provided that sufficient knee range is available for performance of ADL, this outcome measure is a poor sole criterion upon which to judge treatment efficacy in the post-acute phase. Frost et al. (2002 A) compared two home-based programmes – usual care (for example, ROM exercises, quadriceps, and hamstrings strengthening) versus functional exercises (rising from a chair, lifting the leg onto a step, and walking) – that commenced immediately after hospital discharge. At the one-year follow-up assessment there was no difference between groups in 10 m walking speed, pain, knee flexion range, or leg extensor power.
All three of the above studies used intention-to-treat analysis; one study employed therapist blinding (Frost et al. 2002A) and another, partial blinding (Kramer et al. 2003 A), and subjects were randomly allocated to groups. Losses to follow-up were 3 % (Rajan et al. 2004A), 23%(Kramer et al. 2003A), and 43%(Frost et al. 2002A), and all studies described reasons for drop-out. Very few adverse events occurred using the exercises prescribed in these studies. According to the principles of evidence-based practice (Herbert et al. 2005 A/R), the Physiotherapy Evidence Database (PEDro) assigned the following scores to each of the studies: Frost et al. 6/10; Kramer et al. 6/10; and Rajan et al. 7/10; indicating that these studies all provide a moderate level of evidence. It can be concluded that patient outcomes one year post-TKA are not affected by location of rehabilitation delivery (out-patient physiotherapy clinic versus home) or type of exercise (usual versus functional). However, loss to follow-up may be affected by the level of supervision provided by the physiotherapist (out-patient attendance or phone call monitoring versus no monitoring). Larger trials, which provide a greater power to detect small differences in outcome measures, may necessitate revision of these conclusions. Patient outcomes at one year post-TKA indicate that although significant improvements were evident compared to before surgery, there is still a residual level of pain, disability, and loss of knee flexion range; and that patients only just attain the lower limits of age-matched normal function, for example walking speed. A lack of sufficient exercise intensity during rehabilitation may partly contribute to these shortfalls in recovery, but it was not possible to calculate exercise dosage from these trials since exercise intensity was largely patient determined or else it was not described.
QUESTION 8
What is the evidence supporting later post-discharge rehabilitation?
One RCT only (Moffet et al. 2004 A) has examined the effect of commencing rehabilitation later in the post-discharge phase. Ability to exercise in this stage would be anticipated to be greater than in the early post-acute phase, when anaemia, pain, oedema, and residual effects of anaesthesia can cause significant limitation. Moffet et al. (2004 A) employed intention-to-treat analysis: blinding of evaluators; random allocation of subjects; and had only ∼10 % loss to follow-up, with all drop-outs being described, thus providing a moderate to strong level of evidence (PEDro score 7/10). Two months after TKA, patients were randomised to either usual care (strength training, ROM exercise, ice, gait retraining; 26 % also received home visits) or to an intensive 12-week supervised physiotherapy programme, which also included the usual care components. Intensive sessions included strength (for example, maximal isometric contractions of quadriceps and hamstrings; functional exercises such as sit-to-stand and stairs) and endurance exercise training (walking or cycling at 60– 80 % of maximum predicted heart rate for up to 20 min.). Exercise intensity was progressed as required, however, while number of repetitions was reported, intensity of strength training was difficult to assess from the data provided. No adverse events from treatment occurred. At six months post-TKA, patients in the intensive exercise group had increased their 6MWT by 31 % (93 m), compared to 25 % (72 m) increase in the usual care group; a significant effect size between interventions of ∼9 %. Significant treatment effect differences of a similar magnitude were evident in the WOMAC subscales of pain, stiffness, and difficulty in performing ADL. One year after TKA, patients in the intensive group tended to have a higher 6 min. Walk Test distance (P = 0.06; 400 m or ∼1.1 m·s−1, which placed them at the lower limit of normal for their age) than the control group (370 m; 1.03 m·s−1), and both groups had similar levels of pain, stiffness, and difficulty performing tasks. This study demonstrates that more intensive rehabilitation, commenced in the later post-acute phase, results in greater improvements in walking speed at six months post-TKA (and probably also at 12 months, given the near statistical significance and relatively low subject number). Therefore, usual care physiotherapy after TKA probably provides less than optimal stimuli, and patients could likely make further significant gains if sufficiently challenged in the post-discharge rehabilitation period. Further, the authors suggest that increasing the exercise intensity and prolonging the programme may yield greater treatment effects. If so, this not only has important functional relevance for the patient, but also has implications for the progression or retardation of common co-morbidities such as hypertension and type 2 diabetes.
POST-DISCHARGE REHABILITATION FOR MRS JM
Mrs JM has similar co-morbidities (HT, diabetes, cardiac disease) and is of a similar age to the patients in the Moffet et al. (2004 A) study. Her scores for each of the WOMAC subscales are two- to three-fold higher than those reported at two months post-TKA, and are anticipated to improve considerably after surgery. Ideally, Mrs JM’s early post-acute rehabilitation will be conducted from home; however, a retrospective review of effects of the co-morbidities of HT, diabetes, and obesity (all of which Mrs JM suffers from) in 959,839 patients after arthroplasty found that each of the co-morbidities was an independent predictor of increased post-operative complications and non-homebound discharge (Jain et al. 2005A). Additionally, achievement of rehabilitation goals by Mrs JM may be slowed by the presence of OA in her left knee (unoperated). For example, progression from a walking aid to independent ambulation, or the recovery and improvement of walk speed, may be delayed by poor ipsilateral or contralateral joint dysfunction. Certainly, with respect to the latter, our data demonstrate that 15-m walk and TUG times are slower in patients with a knee or hip replacement awaiting further surgery for other joints than in patients with knee or hip replacement who are not (Naylor et al. 2006a A).
Based on the evidence from theRCTsdiscussed above, in the early post-acute phase, Mrs JM will be prescribed an exercise programme that includes ROM and strength exercises (including functional exercise), and gait retraining; and she will receive advice regarding management of oedema and pain. Mrs JM will remain relatively anaemic (Hb 105 g·l−1) at discharge, which may result in mild fatigue, dizziness, and dyspnoea during more demanding submaximal exercise, as a consequence of lower arterial oxygen content. This, coupled with pain, oedema, and the associated muscle inhibition, will reduce the exercise intensity that Mrs JM can undertake in this early period. In addition, given Mrs JM had poor pre-operative control of her diabetes (indicated by the HbA1c), she may experience more difficulty controlling her BGL in the post-acute phase consequent to reduced activity, stress, and hospitalisation. Even so, current opinion (Sigal et al. 2004 A) is that light- or moderate-intensity exercise should not be postponed in those with type 2 diabetes, even if BGL exceeds ∼17 mmol·l−1 (300 mg·dl−1), unless the patient feels unwell and has urinary or blood ketones. We anticipate improved blood glucose control in this case, following the review by the endocrinologist in hospital and the consequent addition of gliclazide to Mrs JM’s usual metformin. Advice from a diabetes educator and a dietician will also enhance her management. Her programme can be conducted at home, with a weekly phone call from her physiotherapist to assess her ability to complete the exercises, to advise on exercise progression, and to monitor potential complications. In the later post-discharge phase, Mrs JM will attend out-patient physiotherapy for a more intensive programme. The programme will commence once oedema and pain have subsided; probably about six to eight weeks post-surgery, and will build upon the gains made with therapy in the acute period. Additionally, based on our recent audit of acute and short-term outcomes following TKA (Naylor et al. 2005 A), we anticipate that Mrs JM will have recovered to ∼90 % of her pre-operative Hb (∼125 g·l−1) by about the sixth week post-TKA. Given the presence of type 2 diabetes mellitus, hypertension, and IHD, current recommendation (Sigal et al. 2004 A) is that it would be prudent to have Mrs JM formally assessed for cardiovascular risk prior to commencing more intense exercise (if not done comprehensively pre-operatively).
Following individual evaluation and exercise prescription, her programme can be undertaken in a group setting, which may be a more cost-effectiveway to deliver more intense, supervised rehabilitation, and may enhance motivation. The programme will include lower limb strength training, functional exercises to promote strength and balance, stretches, and either cycling or walking for local muscular and whole-body endurance. Intensity will be monitored by heart rate and rating of perceived exertion (the latter particularly, if any autonomic neuropathy is suspected or demonstrated), and the number of repetitions and sets of each strength exercise, and the load and duration of endurance exercises will be recorded. As previously stated, the current evidence does not provide sufficient detail to determine exercise dosage for resistance training; hence the following suggestions are based upon research drawn from other sources, and are subject to change when further specific evidence regarding resistance training afterTKAis published. The intensity of resistance exercise will be gradually increased as tolerated, beginning with one set of 10–15 repetitions (not to fatigue) twice per week, and over a number of weeks progressing to three sets of eight repetitions at a 10 RM(repetition maximum) load up to three times per week. The latter is recommended for individuals with type 2 diabetes, to assist with improving metabolic control, for example lowering HbA1c (Sigal et al. 2004 A) – a very desirable outcome in Mrs JM. Resistance exercise is also recommended for patients with OA; however, it is suggested that muscles should not be exercised to fatigue (American Geriatrics Society Panel on Exercise and Osteoarthritis 2001 A). Hence, resistance training for the left leg (knee OA) will be conducted at a lower load and not to fatigue (for example, eight to 10 repetitions at 15 RM load) and will be changed to isometric exercise if the left knee becomes unstable or acutely inflamed. Endurance exercise (walking or cycling) will be commenced at 50 % of maximum heart rate for five to 10 minutes at least every second day, and progressed as tolerated to a weekly dose of 150 minutes at 50–70 % of maximum heart rate (Sigal et al. 2004 A). Based on the results of Moffet et al. (2004 A), Mrs JM can expect to be walking ∼30 % further in a 6MWT after six months; perhaps even more quickly if she has a more intense exercise programme that is continued for a longer period (depending on the degree of limitation from her left knee OA). The ability to undertake both sustained aerobic and resistance exercise is important in addressing Mrs JM’s co-morbidities of obesity, type 2 diabetes, HT, and IHD, and in accomplishing a full return to her ADL (including negotiation of 18 stairs at home) and leisure activities (lawn bowls). In addition, consultation with a diabetes educator and a dietician are recommended for Mrs JM.
Thus it appears that most rehabilitation programmes finish just when the patient is becoming more capable of exercising with greater intensity. The incorporation of more challenging (more intense) exercise may address the deficits in gait speed, muscle strength, and quality of life evident several months to years after TKA (see Introduction).
Given the common occurrence of co-morbidities in patients who undergo TKA, a more protracted exercise programme, which included both strength and endurance components, would be anticipated to have important health and financial benefits.
However, given the paucity of RCTs and the holistic nature of the existing post-acute physiotherapy RCTs, there is little evidence to suggest what the optimal exercise programme after TKA might comprise.
IMPACT OF SURGICAL FACTORS ON LONGER TERM RECOVERY
QUESTION 9
Do prosthesis design and surgical choice impact on rehabilitation or functional recovery? Despite myriad investigations concerning efficacy of TKA, there is significant variation in the prostheses used and surgical decisions made. In other words, despite substantial evidence supporting the intervention, best surgical practice in this field is yet to be recognised. The need to re-align the knee to a neutral mechanical axis and balance the soft tissue is generally agreed upon; some of the issues that remaid debated in the literature include cemented versus uncemented implants, the role of the posterior cruciate ligament, mobile versus fixed bearing, and whether or not to resurface the patella.
Cemented versus uncemented fixation
Mrs JM underwent a cemented TKA. Cemented TKA remains the standard to which alternative forms of fixation need to be compared (Insall et al. 1976 A; Jones et al. 2005 A; Rodriguez et al. 2001 A). In Australia, cemented TKA make up almost 50 % of procedures, while uncemented and hybrid implants comprise 25 % each (Australian Orthopaedic Association National Joint Replacement Registry 2004). Uncemented fixation has the theoretical advantage of osseointegration, which may have implications for longevity, infection, and future bone loss (Diduch et al. 1997 A), while cemented implants have a significant cost benefit. In general terms, failure of uncemented implants has been mainly on the tibial and patella surfaces. Many early designs showed pain scores that were slower to improve than in their cemented counterparts, and had higher revision rates (Duffy et al. 1998A; Ritter 2001A). Newer implant designs may have overcome these problems; however, long-term results are yet to be realised. To date there is no literature examining the impact of weight bearing on early and late fixation in cemented or uncemented prostheses.
Cruciate versus no cruciate
While most current prostheses sacrifice the ACL, controversy remains regarding the PCL. Some argue that preservation of the PCL aids in improving the stability, kinematics, and mechanics of the knee replacement and avoids extra bone resection (Rand 1996 A). Those in favour of excision argue that the PCL is not normal in arthritic knees and that its excision allows improved balancing and correction of deformity (Hirsch et al. 1994 A), as well as more consistent and predictable kinematics (Dennis et al. 1996 A; Dennis et al. 1998 A). Excellent clinical results have been shown with both PCL-retaining and -sacrificing TKAs. Nevertheless, there remain significant differences in the kinematics between normal and replaced knees, and much of this gait abnormality is thought to be related to cruciate deficiency. This, combined with senile muscle weakness and prolonged disability, may further reduce the ability of patients to perform activities, including rehabilitative activities, following TKA.
Two options are available to improve stability and kinematics following resection of the ACL, PCL, or both. One option is to increase the congruity of the polyethylene with anterior and posterior lips, to prevent translation of the tibia relative to the femur.
The other option is for the surgeon to introduce a cam-and-post mechanism, which prevents posterior translation of the tibia relative to the femur. Mrs JM had a posterior stabilised cam-and-post type implant. It is important to recognise that neither PCL retaining nor -substituting implants provide varus or valgus stability, and they both require intact collaterals for stability.
A recent RCT (Straw et al. 2003 A) examined the effect of the PCL in total knee arthroplasty. Patients were randomised to retention or excision of the PCL. There were four groups: (a) PCL retaining and standard implants; (b) PCL released and standard implants; (c) PCL excised and standard implants; (d) PCL excised and posterior substituting implants. There was no difference in groups (a), (c) and (d) with regards to pain scores, range of motion, knee scores, or functional scores. Patients in group (b), with retaining implants and a released PCL, did significantly worse than the other three groups in terms of knee scores and function. The posterior stabilised group (d) had the highest functional scores, walking distance, and stair climbing. The poorest range of motion was in group (a), suggesting tightness in flexion with PCL retention. In terms of clinical stability, posterior stabilised (d) were the most stable, while the excised group (c) were the most lax in the anteroposterior plane; this was not statistically significant, however. There was no difference between groups in terms of mediolateral stability. Follow-up averaged 3.5 years and as such the issue of long-term wear could not be examined.
Integrity of the collateral ligaments
Release of the collateral structures is required during TKA when the gaps created for the implants in flexion and extension are not rectangular. If left asymmetrical, this can lead to asymmetric forces on the medial or lateral sides of the knee and potentially cause pain, instability, poor function and early wear. Creating equal gaps requires correct bony alignment as well as appropriate release of the soft tissues. In the varus knee, medial structures tend to be tight, while in the valgus knee, it is the lateral structures that become tight. The contributing structures will depend on whether the knee is tight in an extended or flexed position. On the medial aspect, the medial collateral ligament and postero-medial capsule may require releasing to balance the knee (Whiteside 1995A). On the lateral aspect, the lateral collateral, popliteal tendon, iliotibial band and capsule may need releasing for balance (Whiteside 1999 A). Mrs JMrequired release of the medial collateral ligament to balance the knee. Occasionally in severe deformity, the opposite side attenuates (for example, medial structures in a valgus knee), thus, requires attention. If so, surgical reconstruction of the ligament is performed, a more constrained form of implant is used, or both. Instability following collateral release does not occur provided that the mechanical axis of the leg has been corrected with the surgery. Ligament releases still leave peripheral attachments and other soft tissue connections, such as periosteum or capsular tissue, which allow the released ligaments to function (Whiteside 2005 A). Ongoing clinical instability, perhaps detected by the therapist if not reported by the patient, usually occurs in the presence of overall limb malalignment, inadequate soft tissue release, or inadvertent transection of ligamentous structures. By and large, collateral release should not impede functional recovery or rehabilitation.
Fixed versus mobile bearing implants
While fixed bearing implants yield excellent results, mobile bearing prostheses were introduced to try and improve wear characteristics, range of motion, and longevity. These implants have dual articulation, with a highly conforming articular surface between the femur and the polyethylene insert. Many designs exist and they vary in the degree of movement allowed between the polyethylene and the base plate. One long-term non-randomised study reported similar clinical and prosthesis survivorship results to fixed bearing implants (Buechel 2002 A), but the impact of activity per se, either early or late, was not addressed. It is tempting to speculate that, given the equivalent prosthesis survivorship across the two designs, neither activity level nor type of activity impacts on long-term functional recovery. However, non-randomised allocation of patients to the varying prosthetic designs may contribute to this; thus, RCTs are ideally needed to confirm this notion. It is also worth noting that trials subjecting the same prostheses to differing long-term in vivo mechanical loading (such as functional and exercise loads) have not been conducted.
Patella resurfacing versus non-resurfacing
Controversy remains over whether or not to resurface the patella. Mrs JM had a cemented patella resurfacing. Many of the early problems with the patellofemoral joint have been addressed by improving the characteristics of the femoral component (Andriacchi et al. 1997 A) and, as such, much of the older literature may not be relevant today. Ongoing anterior knee pain is the reason for considering resurfacing, while complications including patella fracture, extensor mechanism disruption, and loosening are reasons to avoid this option routinely. Resurfacing of the patella is generally agreed upon in inflammatory arthritis, patella maltracking, eburnated bone on the patella, preoperative anterior knee pain, and crystalline deposition disease (Kajino et al. 1997A; Kim et al. 1999A). Studies in patients with bilateral arthroplasty with only one side resurfaced have not shown significant differences (Keblish et al. 1994 A). While there is equivocal evidence from RCTs (Barrack et al. 2001 A;Wood et al. 2002 A), a recent review (Holt & Dennis 2003 R) concluded that, although patient selection is critical to the decision to resurface the patella, unresurfaced patellae deteriorate over time and secondary resurfacing is associated with greater residual patellofemoral pain. This was reiterated by Jones et al. (2005 R), who also concluded that patella resurfacing is likely to improve outcomes, including long-term pain-free patella function. From the therapist’s perspective, knowledge of whether or not the patella was resurfaced may help explain ongoing or residual anterior knee pain, or even pain emerging within a few months to years of the TKA procedure. To our knowledge, there are no context-specific data available to guide the therapist in terms of what, if any, lower limb exercises are preferred in the presence or absence of patella resurfacing.
SUMMARY
The choices that surgeons face when undertaking TKA are manifold. Unfortunately, well constructed RCTs are not available to answer many of the debates that remain, particularly in relation to TKAs’ relevance to rehabilitation. However, from a surgeon’s perspective, there is little doubt that good alignment and good balance are the most important features in providing patients with a well-performing, long-lasting joint replacement. Provided these principles are adhered to, and once best practice rehabilitation is identified, we assume at this stage that post-operative physiotherapy and rehabilitation should not be substantially affected by variations in surgical hardware and technique. Having said that, note that the more cognisant the physiotherapist is of each patient’s surgical particulars, the less risk there is of their doing harm, and the better placed they are to set pragmatic rehabilitation goals.
REFERENCES
Aarons H, Hall G, Hughes S, Salmon P (1996) Short-term recovery from hip and knee arthroplasty.
Journal of Bone and Joint Surgery 78B: 555–558.
Ackerman IN, Bennell KL (2004) Does pre-operative physiotherapy improve outcomes from lower limb joint replacement surgery? A systematic review. Australian Journal of Physiotherapy 50: 25–30.
Ackerman IN, Graves SE, Wicks IP, Bennell KL, Osborne RH (2005) Severely compromised quality of life in women and those of lower socioeconomic status waiting for joint replacement surgery. Arthritis & Rheumatism 53: 653–658.
American Geriatrics Society Panel on Exercise and Osteoarthritis (2001) Exercise prescription for older adults with osteoarthritis pain: consensus practice recommendations. Journal of the American Geriatrics Society 49: 808–823.
Andriacchi TP, Yoder D, Conley A et al. (1997) Patellofemoral design influences function following total knee arthroplasty. Journal of Arthoplasty 12: 243.
Australian Orthopaedic Association National Joint Replacement Registry (2004) Annual Report Adelaide: Australian Orthopaedic Association.
Avramidis K, Strike PW, Taylor PN, Swain ID (2003) Effectiveness of electric stimulation of the vastus medialis muscle in the rehabilitation of patients after total knee arthroplasty.
Archives of Physical Medicine and Rehabilitation 84: 1850–1853.Bachmeier CJM, March L, Cross M,
Lapsely H, Tribe K, Courtenay B et al. (2001) A comparisonof outcomes in osteoarthritis patients undergoing total hip and knee replacement surgery. Osteoarthritis and Cartilage 9: 137–146.
Barrack RL, Bertot AJ, Wolfe MW, Waldman DA, Milicic M, Myers L (2001) Patellar resurfacing
in total knee arthroplasty: a prospective, randomized, double-blinded study with five to seven years of follow-up. Journal of Bone and Joint Surgery 83A: 1376–1381.
Barry S, Wallace L, Lamb S (2003) Cryotherapy after total knee replacement: a survey of current practice. Physiotherapy Research International 8: 111–120.
Bellamy N, BuchananW, Goldsmith C, Campbell J, StittL(1988)Validation study ofWOMAC:
a health status instrument for measuring clinically-important patient-relevant outcomes
following total hip or knee arthroplasty in osteoarthritis. Journal of Orthopaedic Rheumatology
1: 95–108.
Benedetti MG, Catani F, Bilotta TW, Marcacci M, Mariani E, Giannini S (2003) Muscle activation pattern and gait biomechanics after total knee replacement. Clinical Biomechanics 18: 871–876.
Benick RA, Backus SI, Kroll MA, Ganz SB, MacKenzie CR (2004) Knee flexion and functional ambulatory status following unilateral total knee arthroplasty. Topics in Geriatric Rehabilitation 20: 308.
Berth A, Urbach D, Awiszus F (2002) Improvement of voluntary quadriceps muscle activation after total knee arthroplasty. Archives of Physical Medicine and Rehabilitation 83: 1432– 1436.
Bischoff HA, Roos EM (2003) Effectiveness and safety of strengthening, aerobic, and coordination exercises for patients with osteoarthritis. Current Opinion in Rheumatology 15: 141–144.
Bozic KJ, Durbhakula S, Berry DJ, Naessens JM, Rappaport K, Cisternas M et al. (2005) Differences in patient and procedure characteristics and hospital resource use in primary and revision total joint arthroplasty. Journal of Arthroplasty 20: 17–25.
Brosseau L, Davis J, Drouin H, Milne S, Noel M, Robinson VA et al. (2006) Continuous passive motion following total knee arthroplasty. Cochrane Library 1 http:// www.thecochranelibrary.comCD004260.
Brunenberg DE, van Steyn MJ, Sluimer JC, Bekebrede LL, Bulstra SK, Joore MA (2005) Joint recovery programme versus usual care: an economic evaluation of a clinical pathway for joint replacement surgery. Medical Care 43: 1018–1026.
Buechel FF Sr (2002) Long-term follow-up after mobile-bearing total knee replacement. Clinical Orthopaedics and Related Research 404: 40–50.
Denis M, Moffet H, Caron F, Ouellet D, Paquet J, Nolet L (2006) Effectiveness of continuous passive motion and conventional physical therapy after total knee arthroplasty: a randomized clinical trial. Physical Therapy 86: 174–185.
Dennis DA, Komistek RD, Hoff WA, Gabriel SM (1996) In vivo knee kinematics derived using an inverse perspective technique. Clinical Orthopaedics and Related Research 331: 107–117.
Dennis DA, Komistek RD, Colwell CE Jr, Ranawat CS, Scott RD, Thornhill TS et al. (1998) In vivo anteroposterior femorotibial translation of total knee arthroplasty: a multicenter analysis. Clinical Orthopaedics and Related Research 356: 47– 57.
Diduch DR, Insall JN, Scott WN, Font-Rodriguez D (1997) Total knee replacement in young, active patients: long-term follow-up and functional outcome. Journal of Bone and Joint Surgery 79A: 575–582.
Dixon T, Shaw M, Ebrahim S, Dieppe P (2004) Trends in hip and knee joint replacement: socioeconomic inequalities and projections of need. Annals of Rheumatic Diseases 63: 825–830.
Dowsey M, Kilgour ML, Santamaria NM, Choong FM (1998) Clinical pathways in hip and knee arthroplasty: a prospective randomized controlled study. Medical Journal ofAustralia
170: 59–62.
Duffy GP, Berry DJ, Rand JA (1998) Cement versus cementless fixation in total knee arthroplasty.
Clinical Orthopaedics and Related Research 356: 66–72.
Ethgen O, Bruyere O, Richy F, Dardennes C, Reginster JY (2004) Health-related quality of life in total hip and total knee arthroplasty. Journal of Bone and Joint Surgery 86A: 963– 971.
Erler K, Anders C, Fehlberg G, Neumann U, Brucker L, Scholle HC (2001) Measurements of results of a special hydrotherapy during in-patient rehabilitation after implantation of a total knee arthroplasty. Zeitschrift f¨ur Orthop¨adie und ihre Grenzgebiete 139: 352–358.
Fortin PR, Penrod JR, Clarke AE, St-Pierre Y, Joseph L, Belisle P et al. (2002) Timing of total joint replacement affects clinical outcomes among patients with osteoarthritis of the hip or knee. Arthritis & Rheumatism 46: 3327–3330.
Fortin PR, Clarke AE, Liang JL, Tanzer M, Ferland D et al. (1999) Outcomes of total hip and knee replacement: preoperative functional status predicts outcomes at six months after surgery. Arthritis & Rheumatism 42: 1722–1728.
Fransen M, Crosbie J, Edmonds J (2003) Isometric muscle force measurement for clinicians treating patients with osteoarthritis of the knee. Arthritis & Rheumatism 49: 29–35.
Fransen M, Crosbie J, Edmonds J (2001) Physical therapy is effective for patients with osteoarthritis of the knee: a randomized controlled clinical trial. Journal of Rheumatology 28: 156–164.
Fransen M, McConnell S, BellM(2002) Therapeutic exercise for people with osteoarthritis of the hip or knee: a systematic review. Journal of Rheumatology 29: 1737–1745.
Frost H, Lamb SE, Robertson S (2002)Arandomized controlled trial of exercise to improve mobility
and function after elective knee arthroplasty. Feasibility, results and methodological difficulties. Clinical Rehabilitation 16: 200–209.
Ganz SB, Benick RA (2004) A comparison of functional recovery following unilateral and bilateral total knee arthroplasty. Topics in Geriatric Medicine 20: 310.
Ganz SB, Ranawat CS (2004) Efficacy of formal knee flexion exercises on the achievement of functional milestones following total knee arthroplasty. Topics in Geriatric Medicine 20: 311.
Gibbons CER, Solan MC, RickettsDM,PattersonM(2001) Cryotherapy compared with Robert Jones bandage after total knee replacement: a prospective randomized trial. International Orthopaedics 25: 250–252.
Gotlin RS, Hershkowitz S, Juris PM, Gonzalez EG, Scott WN, Insall JN (1994) Electrical
stimulation effect on extensor lag and length of hospital stay after knee arthroplasty.
Archives of Physical Medicine and Rehabilitation 75: 957–959.
Gur H, Cakin N, Akova B, Okay E, Kucukoglu S (2002) Concentric versus combined
concentric-eccentric isokinetic training: effects on functional capacity and symptoms in
patients with osteoarthrosis of the knee. Archives of Physical Medicine and Rehabilitation
83: 308–316.
Hamdan TA, Al-Essa KA (2003) Manipulation under anaesthesia for the treatment of frozen
shoulder. International Orthopaedics 27: 107–109.
Haug J, Wood LT (1988) Efficacy of neuromuscular stimulation of the quadriceps femoris
during continuous passive motion following total knee arthroplasty. Archives of Physical
Medicine and Rehabilitation 69: 423–424.
Healy WL, Seidman J, Pfeifer BA, Brown DG (1994) Cold compressive dressings after total
knee arthroplasty. Clinical Orthopaedics and Related Research 299: 143–146.
Heck D, Robinson R, Partridge C, Lubitz R, Freund D (1998) Patient outcomes after knee
replacement. Clinical Orthopaedics and Related Research 356: 93–110.
Herbert, R, Jamtvedt, G, Mead, J, Hagen, KB (2005) Practical Evidence-Based Physiotherapy
Edinburgh: Elsevier.
Hirsch HS, Lotke PA, MorrisonLD(1994) The posterior cruciate ligament in total knee surgery.
Save, sacrifice, or substitute? Clinical Orthopaedics and Related Research 309: 64–68.
Holt G, Dennis D (2003) The role of patella resurfacing in total knee arthroplasty. Clinical
Orthopaedics and Related Research 1: 76–83.
Insall JN, Ranawat CS, Scott WN, Walker P (1976) Total condylar knee replacement: preliminary
report. Clinical Orthopaedics and Related Research 120: 149–154.
Ivey M, Johnston RV, Uchida T (1994) Cryotherapy for postoperative pain relief following
knee arthroplasty. Journal of Arthroplasty 9: 285–290.
Jain NB, Guller U, Pietrobon R, Bond TK, Higgins LD (2005) Comorbidities increase complication
rates in patients having arthroplasty. Clinical Orthopaedics and Related Research
435: 232–238.
Jones DL,Westby MD, Greidanus N, Johanson NA, Krebs DE, Robbins L et al. (2005) Update
on hip and knee arthroplasty: current state of evidence. Arthritis & Rheumatism 53: 772–
780.
Kajino A, Yoshino S, Kameyama S, Kohda M, Nagashima S (1997) Comparison of results of bilateral total knee arthroplasty with and without patellar replacement for rheumatoid arthritis: a follow-up note. Journal of Bone and Joint Surgery 79A: 570.
Keblish PA, Varma AK, Greenwald AS (1994) Patellar resurfacing or retention in total knee arthroplasty: a prospective study of patients with bilateral replacements. Journal of Bone and Joint Surgery 76B: 930.
Kennedy DM, Stratford PW, Wessel J, Gollish JD, Penney D (2005) Assessing stability and change of four performance measures: a longitudinal study evaluating outcome following total hip and knee arthroplasty. BMC Musculoskeletal Disorders 6(3).
Kim BS, Reitman RD, Schai PA, Scott RD (1999) Selective patellar nonresurfacing in total knee arthroplasty: 10 year results. Clinical Orthopaedics and Related Research 367: 81.
Kramer JF, Speechley M, Bourne R, Rorabeck C, Vaz M. (2003) Comparison of clinic- and home-based rehabilitation programs after total knee arthroplasty. Clinical Orthopaedics and Related Research 410: 225–234.
Lachiewicz PF (2000) The role of continuous passive motion after total knee arthroplasty. Clinical Orthopaedics and Related Research 1: 144–150.
Lamb SE, Frost H (2003) Recovery of mobility after knee arthroplasty. Expected rates and influencing factors. Journal of Arthroplasty 18: 575–581.
Lingard EA, Bervan S, Katz JN, Kinemax Outcomes Group (2000) Management and care of patients undergoing total knee arthroplasty: variations across different health care settings. Arthritis Care and Research 13: 129–136.
Lorentzen JS, Petersen MM, Brot C, Madsen OR (1999) Early changes in muscle strength after total knee arthroplasty. Acta Orthopedica Scandinavica 70: 176–179.
March LM, Cross M, Tribe KL, Lapsley HM, Courtenay BG, Cross MJ et al. (2004) Two knees or not two knees? Patient costs and outcomes following bilateral and unilateral total knee joint replacement surgery for OA. Osteoarthritis Cartilage 12: 400–408.
MarchLM,Cross MJ, LapsleyH, Brnabic AJ,Tribe KL, BachmeierCJMet al. (1999) Outcomes
after hip or knee replacement surgery for osteoarthritis. Medical Journal of Australia 171:
235–238.
Martin SS, Spindler KP, Tarter JW, Detwiler KB (2002) Does cryotherapy affect intraarticular
temperature after knee arthroscopy? Clinical Orthopaedics and Related Research 1: 184–
189.
McAuley JP, HarrerMF, Ammeen D, EnghGA(2002) Outcome of knee arthroplasty in patients
with poor pre-operative range of motion. Clinical Orthopedics and Related Research 404:
203–207.
McDonald S, Hetrick S, Green S (2004) Pre-operative education for hip or knee replacement.
Cochrane Library 1 http://www.thecochranelibrary.com CD003526.
Milne S, Brosseau L, Robinson V, Noel MJ, Davis J, Drouin H et al. (2003) Continuous
passive motion following total knee arthroplasty. Cochrane Library 2 http://
www.thecochranelibrary.com CD004260.
Mitchell C, Walker J, Walters S, Morgan AB, Binns T, Mathers N (2005) Costs and effectiveness
or pre- and post-operative physiotherapy for total knee replacement: randomized
controlled trial. Journal of Evaluation in Clinical Practice 11: 283–292.
Mizner RL, Stevens JE, Snyder-Mackler L (2003)Voluntary activation and decreased force production
of the quadriceps femoris muscle after total knee arthroplasty. Physical Therapy
83: 359–365.
Mizner RL, Petterson SC, Stevens JE, Axe MJ, Snyder-Mackler L (2005) Preoperative quadriceps
strength predicts functional ability one year after total knee arthroplasty. Journal of
Rheumatology 32: 1533–1539.
Moffet H, Collet J-P, Shapiro SH, Paradis G, Marquis F, Roy L (2004) Effectiveness of intensive
rehabilitation on functional ability and quality of life after first total knee arthroplasty: a
single-blind randomised controlled trial. Archives of Physical Medicine and Rehabilitation
85: 546–556.
Munin MC, Rudy TE, GlynnNW, Crossett LS, Rubash HE (1998) Early inpatient rehabilitation
after elective hip or knee arthroplasty. Journal of the American Medical Association 279:
847–852.
Australian Bureau of Statistics (1995) National Health Survey SF-36 Population Norms Canberra:
Australian Bureau of Statistics, Cat. No. 4399.0.
Naylor JM, Ireland JE, Mohammed M (2005) Outcomes Following Primary THR and TKR:
part 1 – acute and short-term outcomes Sydney: Whitlam Joint Replacement Centre,
SSWAHS.
Naylor JM, Fransen M, Ireland JE, Winstanley J (2006a) Outcomes Following Primary THR
and TKR: part 2 – longer-term outcomes. Sydney: Whitlam Joint Replacement Centre,
SSWAHS.
Naylor JM, Harmer AR, Fransen M, Crosbie J, Innes L (2006b) The status of physiotherapy
rehabilitation following total knee replacement in Australia. Physiotherapy Research
International 11: 35–47.
NHMRC and ASBT (2001) Clinical practice guideline on the use of blood components.
http://www.nhmrc.health.gov.au/.
Oldemeadow L, McBurney H, Robertson V (2001) Hospital stay and discharge outcomes after
knee arthroplasty. Journal of Quality and Clinical Practice 21: 56–60.
Ouellet D, Moffet H (2002) Locomotor deficits before and two months after knee arthroplasty.
Arthritis & Rheumatism 47: 484–493.
Paul RG, Bailey AJ (1996) Glycation of collagen: the basis of its central role in the late
complications of ageing and diabetes. International Journal of Biochemistry and Cell
Biology 28: 1297–1310.
Pearson S, Moraw I, Maddern GJ (2000) Clinical pathway management of total knee arthroplasty:
a retrospective comparative study. ANZ Journal of Surgery 70: 351–354.
Petterson SC, Mizner RL, Snyder-Mackler L (2003) Factors that influence six-minute walk in
individuals after total knee arthroplasty. Journal of Geriatric Physical Therapy 26: 50.
Pierson J, Earles D,Wood K (2003) Brake response time after total knee arthroplasty. Journal
of Arthroplasty 18: 840–843.
Rajan RA, PackY, Jackson H, Gillies C, AsirvathamR(2004)Noneed for outpatient physiotherapy
following total knee arthroplasty: a randomized trial of 120 patients. Acta Orthopedica
Scandinavica 75(1): 71–73.
Rand JA (1996) Posterior cruciate retaining total knee arthroplasty. In: Morrey BF (ed.) Reconstructive
Surgery of the Joints Volume 2 (2 edn) New York: Churchill Livingstone, pp.
1401–1408.
Ritter MA, Berend ME, Meding JB, Keating EM, Faris PM, Crites BM (2001) Long-term follow-up of anatomic graduated components posterior cruciate-retaining total knee replacement. Clinical Orthopedics 388: 51–57.
Rodriguez JA, Bhende H, Ranawat CS (2001) Total condylar knee replacement: a 20-year follow-up study. Clinical Orthopaedics and Related Research 388: 10–17.
Roos E (2003) Effectiveness and practice variation of rehabilitation after joint replacement. Current Opinion in Rheumatology 15: 160–162.
Rossi MD, Hasson S (2004) Lower-limb force production in individuals after unilateral total knee arthroplasty. Archives of Physical Medicine and Rehabilitation 85: 1279–1284.
Salmon P, Hall G, Peerbhoy D, Shenkin A, Parker C (2001) Recovery from hip and knee arthroplasty: patients’ perspective on pain, function, quality of life, and well-being up to 6 months postoperatively. Archives of Physical Medicine and Rehabilitation 82: 360–366.
Scarcella JB, Cohn BT (1995) The effect of cold therapy on the postoperative course of total hip and knee arthroplasty patients. American Journal of Orthopedics November: 847– 852.
Segal L, Day SE, Chapman AB, Osborne RH (2004) Can we reduce disease burden from osteoarthritis? An evidence-based priority-setting model. Medical Journal of Australia 180: 11S–17S.
Shields RK, Enloe LJ, Leo KC (1999) Health related quality of life in patients with total hip or knee replacement. Archives of Physical Medicine and Rehabilitation 80: 572–579.
Shumway-Cook A, Brauer S, Woollacott M. (2000) Predicting the probability for falls in community-dwelling older adults using the timed up and go test. Physical Therapy 80: 897–903.
Sigal RJ, Kenny GP, Wasserman DH, Castaneda-Sceppa C (2004) Physical activity/exercise and type 2 diabetes. Diabetes Care 27: 2518–2539.
Skinner J, Weinstein JN, Sporer SM, Wennberg JE (2003) Racial, ethnic, and geographic disparities in rates of knee arthroplasty among Medicare patients. New England Journal of Medicine 349: 1350–1359.
Smith J, Stevens J, Taylor M, Tibbey J (2002) A randomised controlled trial comparing compression
bandaging and cold therapy in postoperative total knee replacement surgery. Orthopaedic Nursing 21: 61–66.
Steffen T, Hacker TA, Mollinger L (2002) Age- and gender-related test performance in community-dwelling elderly people: six-minute walk test, berg balance scale, timed up & go test, and gait speeds. Physical Therapy 82: 128–137.
Stratford PW, Kennedy D, Pagura SM, Gollish JD (2003) The relationship between self-report and performance-related measures: questioning the content validity of timed tests. Arthritis & Rheumatism 49: 535–540.
Straw R, Kulkarni S, Attfield S, Wilton TJ (2003) Posterior cruciate ligament at total knee
replacement. Essential, beneficial or hindrance? Journal of Bone and Joint Surgery 85B: 671–674.
van Essen GJ, Chipcase LS, O’Connor D, Krishnan J (1998) Primary total knee replacement: short-term outcomes in an Australian population. Journal of Quality in Clinical Practice 18: 135–142.
Walsh M, Woodhouse LJ, Thomas SG, Finch E (1998) Physical impairments and functional limitations: a comparison of individuals 1 year after total knee arthroplasty with control subjects. Physical Therapy 78: 248–258.
Wang A, Hall S, Gilbey H, Ackland T (1997) Patient variability and the design of clinical pathways after total hip replacement surgery. Journal of Quality in Clinical Practice 17: 123–129.
Webb JM, Williams D, Ivory JP, Day S, Williamson DM (1998) The use of cold compression dressings after total knee replacement: a randomised controlled trial. Orthopedics 21: 59–61.
Whiteside LA (1995) Ligament balancing and bone grafting in total knee replacement of the varus knee. Orthopedics 18: 117–122.
Whiteside LA (1999) Selective ligament release in total knee arthroplasty of the knee in valgus. Clinical Orthopedics and Related Research 367: 130–140.
Whiteside LA. (2005) Assess and release the tight ligament. In: Bellemans J, Ries MD, Victor J (eds) Total Knee Arthroplasty: a guide to get better performance, pp. 170–176.
Wood DJ, Smith AJ, Collopy D, White B, Brankov B, Bulsara MK (2002) Patellar resurfacing in total knee arthroplasty: a prospective, randomized trial. Journal of Bone and Joint Surgery 84A: 187–193.
Subscrever:
Mensagens (Atom)